Supercapacitors are energy storage devices that store electrical charge via physical adsorption/desorption processes. These occur between an electrode and electrolyte interface without solid-state-diffusion, leading to high power performance [1]. The electrochemical performance of supercapacitors is highly dependent on the characteristics of the electrode materials used in their fabrication [2,3]. Activated carbon has typically been used as an electrode material due to its high specific surface area, sub-nanometer pores, and high electrical conductivity; in addition to having a low cost of material. However, conventional supercapacitors are limited due to their low energy character. Thus, advanced electrode designs comprising high surface areas, well-defined pore structures, and nano-dimensional structures are required to yield supercapacitors with improved energy and power characteristics [4-7].
Roughly 8 million metric tons of coffee beans are produced and consumed by the global beverage industry each year [8]. For each kilogram of beverage coffee produced, approximately 0.91 kg of waste is generated. Coffee waste is classified as a contaminant because the organic matter in soil consumes large quantities of oxygen while decomposing [9]. Therefore, many studies (spread over various fields) have been performed in search of a method for processing waste coffee grounds (WCGs). WCGs are composed primarily of macromolecular cellulose and lignocellulose, which is known to be an exceptional carbon precursor. The molecular structure of lignocellulose includes aromatic hexagonal carbon atoms that can be carbonized via relatively rapid pyrolysis [10]. Accordingly, the use of WCGs as source for carbon and activated carbon has been widely reported [11-14]. Nevertheless, there are few reports on developing nanostructured carbon-based materials from WCGs.
In this study, nanoporous carbon nanosheets (NCNSs) were fabricated from WCGs via simple heating with KOH. NCNSs were easily obtained through a one-step top-down method from WCGs without any additional treatment. The NCNSs exhibited a high specific surface area of 1960.1 m2 g−1 with numerous redox-active heteroatoms such as oxygen, nitrogen, and sulfur. They also included two-dimensional (2D) nanostructures with high aspect ratios (>100). These superior material properties led to good electrochemical performance as supercapacitor electrodes, exhibiting a specific capacitance of ~438.5 F g−1 at a scan rate of 2 mV s−1 with good rate capabilities and exceptional cyclic stability.
2.1. Preparation of carbon materials
The WCGs, from commercial beverage manufacturers (Starbucks®), were dried at 80℃ for 48 h. The dried WCGs were mixed at a weight ratio of 1:1 with KOH (95%; Samchun Pure Chemical, Seoul, Korea) in a mortar and were subsequently pyrolyzed in a tube furnace under N2 gas flow at a heating rate of 10℃ min−1 up to 800℃, and held at this temperature for 2 h. The resulting NCNSs were washed with deionized water and ethanol to remove leftover residues and then were dried in a vacuum oven at 60℃. For a reference sample, the dried WCGs were pyrolyzed under the same conditions in the absence of KOH. The resultant products are referred to as C-WCGs.
The thermal behavior of the WCGs was analyzed via thermogravimetric analysis (TGA; TG 209 F3, NETZSCH, Germany) from room temperature to 800℃ at a heating rate of 10℃ min−1 under N2 gas. Samples morphologies were examined via field-emission scanning electron microscopy (S-4300; Hitachi, Tokyo, Japan) and field-emission transmission electron microscopy (JEM2100F; JEOL, Tokyo, Japan). X-ray diffraction (XRD; DMAX2500; Rigaku, Tokyo, Japan) analysis was carried out via Cu Kα radiation (wavelength λ = 0.154 nm) at 40 kV and 100 mA. Raman spectroscopy (Jobin Yvon, Paris, France) was employed to characterize sample microstructures. The chemical composition of the samples was examined via X-ray photoelectron spectroscopy (XPS; PHI 5700 ESCA; Physical Electronics, Chanhassen, MN, USA) with monochromatic Al Kα radiation (hv = 1486.6 eV). The porous properties of the samples were analyzed using nitrogen adsorption/desorption isotherms that were determined using the surface area and a porosimetry analyzer (ASAP 2020; Micromeritics, Norcross, GA, USA) at −196℃. Brunauer-Emmett-Teller (BET) surface areas were calculated according to the BET theory. The micropore surface areas and mesopore surface areas were obtained via t-plot theory and the Barrett-Joyner-Halenda theory, respectively.
2.3. Electrochemical characterization
Electrochemical properties were measured using a three-electrode system consisting of a working electrode (NCNSs), counter electrode (Pt wire), and reference electrode (Ag/AgCl). The NCNSs and polytetrafluoroethylene (60 wt% dispersion in H2O; Sigma-Aldrich, St. Louis, MO, USA) were mixed in a 9:1 mass ratio and dispersed in ethanol, yielding a homogeneous paste. The ethanol was subsequently evaporated in an oven at 80℃. The resulting mixtures were coated onto nickel mesh (1 × 1 cm). The electrolyte material used was 6 M NaOH and 1 M Na2SO4.
3.1. Thermal decomposition behavior of WCGs
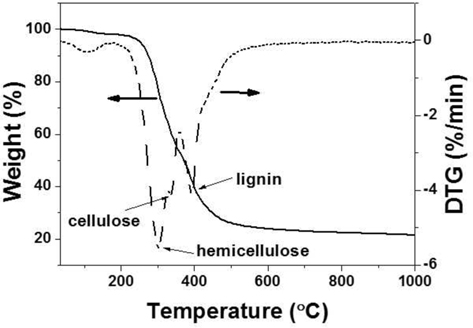
Fig. 1 presents the thermal decomposition behavior of the WCGs. In the first temperature range from ambient to 200℃, a slight weight loss in the TGA curve was attributed to the release of moisture and light volatile materials. The next temperature range from 200℃ to 500℃ was represented by numerous weight losses (ca. 70%) due to the primary pyrolysis of the WCGs and the formation of carbonaceous materials. In this range of the differential thermogravimetry curve, three peaks were observed that were indicative of the decomposition of hemicellulose, cellulose, and lignin. The last temperature range (to over 500℃) exhibited a slight weight loss resulting from the consolidation of carbon structures [15].
3.2. Morphologies and microstructures of NCNSs and C-WCGs
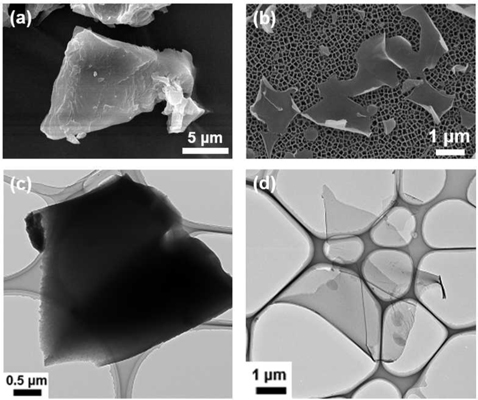
Morphologies of the carbonaceous materials fabricated from WCGs are presented in Fig. 2. C-WCGs exhibited random micron-scale bulk particle shapes (Fig. 2a and c), while the NCNSs exhibited 2D-like nanosheet structures with high aspect ratios (>100), as shown in Fig. 2b and d. The KOH activation process involved three primary mechanisms according to a prior study [16]: 1) the generation of pore structures via redox reactions between various potassium compounds and carbon, 2) the additional development of pore structures via the gasification of carbon, and 3) the expansion of carbon lattices by intercalating metallic K generated during activation into the carbon matrix. Thus, the structural differences between NCNSs and C-WCGs resulted from activation effects in which large quantities of carbon were etched and hexagonal carbon layers were exfoliated via KOH at temperatures above 700℃.
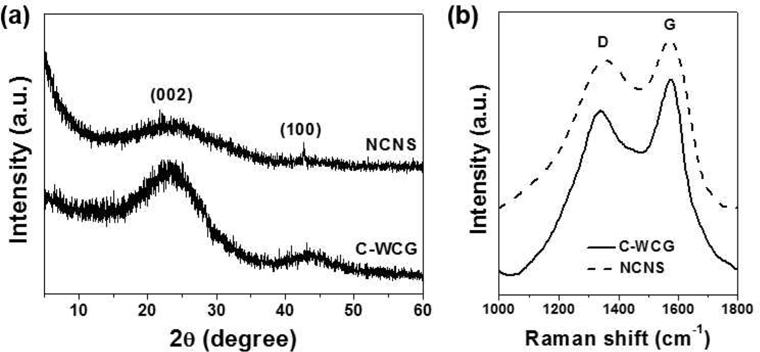
Microstructures of NCNSs and C-WCGs were analyzed via XRD and Raman spectroscopy, as shown in Fig. 3. The XRD pattern of C-WCGs exhibited two broad peaks at 23.3° and 43.3°, corresponding to planar graphite (002), indicating the degree of carbon layer stacking, and (100), indicating the degree of ordered hexagonal carbon structures, respectively (Fig. 3a) [17]. However, the XRD pattern of the NCNSs revealed a very broad graphite (002) peak resulting from carbon layer exfoliation via potassium during the pyrolysis process. By contrast, the Raman spectra of C-WCGs and NCNSs exhibited two distinct peaks centered at 1353 and 1573 cm−1, revealing typical carbon characteristics (Fig. 3b). The
3.3. Chemical structure and porous properties of NCNSs
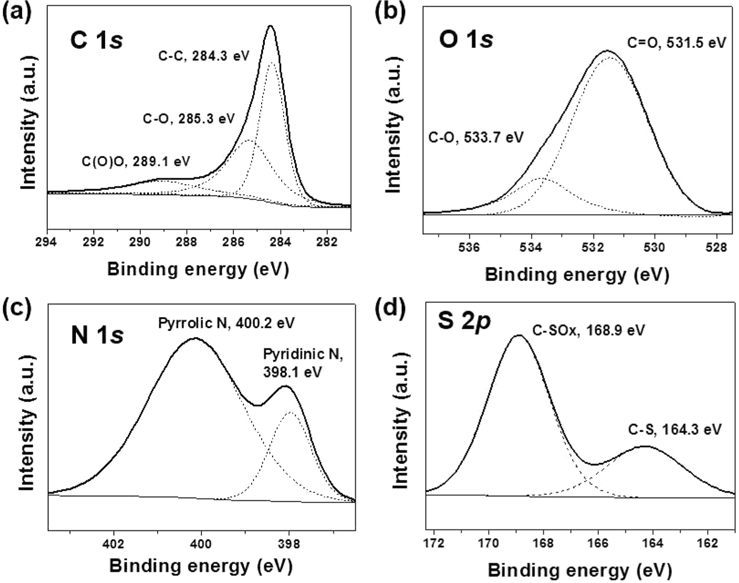
The chemical configuration of NCNSs was characterized via XPS as shown in Fig. 4. In the XPS C 1
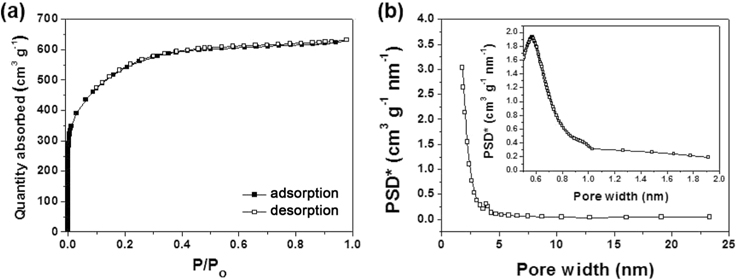
The porous properties of the NCNSs were investigated via nitrogen adsorption/desorption isotherm experiments (Fig. 5). The isotherm curves of the NCNSs were representative of International Union of Pure and Applied Chemistry Type-I compounds, indicating the existence of microporous structures (Fig. 5a). The specific surface area of NCNSs was 1960.1 m2 g−1, and most were micropores (1932.5 m2 g−1) ~0.6 nm in size (Fig. 5b). The texture properties of the NCNSs are summarized in Table 1.
[Table 1.] Nanoporous carbon nanosheet textural properties

Nanoporous carbon nanosheet textural properties
3.4. Electrochemical characteristics of NCNSs
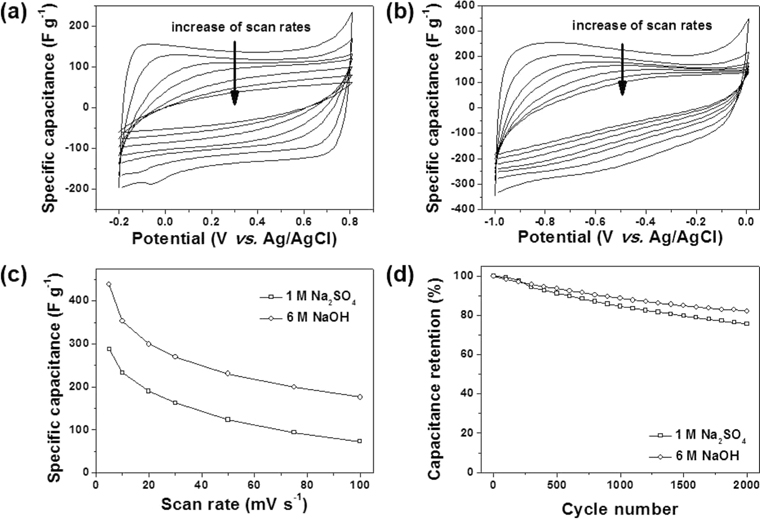
The electrochemical properties of the NCNSs were investigated in aqueous electrolytes (1 M Na2SO4 and 6M NaOH) over the potential range between −0.2 and 0.8 (V vs. Ag/AgCl) and −1 and 0 (V vs. Ag/AgCl), respectively. Fig. 6a reveals cyclic voltammogram (CV) curves in 1 M Na2SO4 aqueous electrolyte. The CV curves exhibited nearly rectangular shapes, indicating ideal capacitive behavior by the physical adsorption and desorption of solvated anions (hydrated SO4− ). Steep slopes of the current changes during switching potentials, indicated a small anion-storage mass-transfer resistance. However, with an increase in scan rates, the rectangular CV curves gradually became oval in shape. By contrast, the CV curves in 6 M NaOH aqueous electrolyte exhibited a continual current increase with decrease in the sweeping potential (Fig. 6b). This difference could be induced by pseudocapacitive charges stored in heteroatoms on the surface of the NCNSs [22-24]. In the 6 M NaOH aqueous electrolyte, hydrated Na+ ions could be charge carriers, in contrast to the 1 M Na2SO4 aqueous electrolyte, of which the charge carriers were hydrated SO4− . Na+ could be stored in oxygen, nitrogen, and sulfur atom sites via pseudocapacitive behavior. The specific capacitance of NCNSs in 6 M NaOH was ~438.5 F g−1, which was much higher than the 287.9 F g−1 observed for 1 M Na2SO4. This capacitance difference supported pseudocapacitance in the 6 M NaOH aqueous electrolyte. Rate performance was investigated at various scan rates (Fig. 6c). As the scan rates were increased, the specific capacitances gradually decreased, and at 100 mV s−1, 176 and 73 F g−1 capacitances were achieved in the 6 M NaOH and 1 M Na2SO4 aqueous electrolytes, respectively. This indicated good rate performance. Additionally, cyclic stability was maintained over 2000 consecutive cycles (Fig. 6d). After 2000 cycles, NCNSs exhibited capacitance retentions of 81.9% and 75.7% in 6 M NaOH and 1 M Na2SO4 aqueous electrolytes, respectively.
NCNSs were fabricated from WCGs via simple heating with KOH. The NCNSs exhibited amorphous carbon structures composed of defective hexagonal carbon structures lacking long-range order, as well as high aspect ratios greater than 100. Numerous heteroatoms such as 16.1 at% oxygen, 2.7 at% nitrogen, and 1.6 at% sulfur were confirmed on the surface of the NCNSs, and were present in various chemical structures. Additionally, the NCNSs yielded a high specific surface area of 1960.1 m2 g−1 with a large quantity of sub-nanometer pores. These unique material properties led to good electrochemical performance as supercapacitor electrodes. A specific capacitance of ~438.5 F g−1, good rate capabilities, and stable cyclic lifetimes to over 2000 cycles were achieved with 6 M NaOH aqueous electrolytes.