Heavy metals such as lead and copper are often found in industrial wastewaters. The acute toxicity of heavy metals can cause various damages to vital internal organs or the central nervous system and lower energy levels. Long-term exposure to heavy metals may result in slowly progressing physical, muscular, and neurological degeneration. Due to the concern regarding the potential risks of heavy metals in aquatic environments, immense attention has been placed on finding technical solutions for removing heavy metals from wastewater.
Various physical and/or chemical treatment technologies such as adsorption, precipitation, complexation, chelation, membrane filtration, ion exchange, reverse osmosis, solvent extraction, etc., have been proposed and applied. However, in many cases, these techniques have been ineffective or impractical due to the low removal efficiency or high operating costs, especially in treating wastewaters with relatively low metal concentrations [1]. As a promising alternative, the application of biologic materials such as bacteria, algae, fungi, seaweeds, and sawdust as a sorbent has been studied over the past few decades [2,3]. The “biosorption” refers to the passive uptake of pollutants from aqueous solutions, often, by the use of non-growing or nonliving biomass [4], and has been known to be highly selective, efficient, relatively cost-effective, and non-hazardous [5-7]. The use of dead or inactive biomass as a biosorbent has many advantages compared to living or active biomass. The dead biomass is not affected by toxic or oligotrophic conditions in wastewaters and is easy to store, operate, and reuse [8,9]. Furthermore, it has been reported that dead biomass can uptake more heavy metals than living biomass [8,10].
In addition to sorption capacity, the biosorbents need to be equipped with mechanical strength, chemical resistivity, and hydraulic permeability for practical applications such as packed or fluidized bed reactors. These requirements can be achieved by the imprisonment of the powdered biomass in a distinct phase with proper size, shape, and rigidity. There are several immobilization techniques reported in the literature, which include 1) adsorption on inert mineral or organic supports such as clay, aluminum oxide, starch, etc.; 2) entrapment in polymeric matrix such as calcium alginate, polyacrylamide, polysulfone, etc.; 3) covalent bonds in vector compounds such as silica gel; or 4) cross-linking with agents such as formaldehyde, divinyl sulfone, formaldehyde, etc. [11]. Among these techniques, immobilization of biomass in a polymer matrix has drawn great attention mainly due to its mechanical and chemical stability. In addition, granule-shaped polymer sorbents can be easily manufactured with customized specifications such as size or biomass contents to meet the requirements of a changing environment. The biomass immobilization can also provide additional advantages such as efficient and effective regeneration for the reuse of the biomass, easier solid-liquid separation, and minimal clogging in continuous flow systems [8].
This study aimed to investigate the thermodynamics, adsorption mechanisms, and kinetics of Pb(II) and Cu(II) biosorption by the immobilized biomass biocarrier beads. For this purpose, the biocarrier beads were fabricated with
2.1. Preparation of Dead Biomass for Immobilized Biocarrier Beads
Indigenous microorganisms were isolated from soil samples collected at a military base contaminated by oils and heavy metals in Daegu, Korea. Out of 16 microbial species, a strain of microorganisms dominant in the colony population, identified as
conduct batch experiments [12].
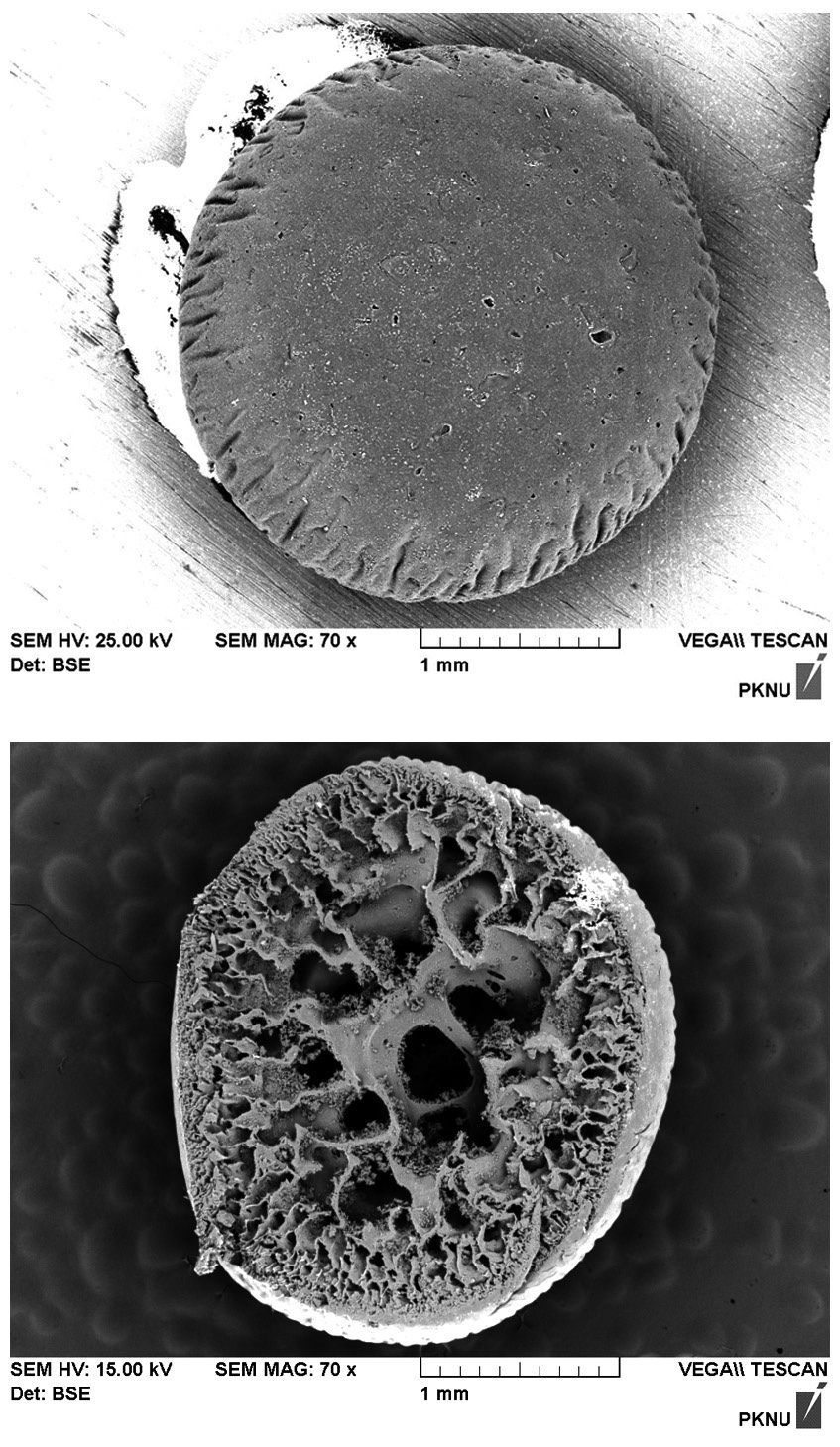
The beads, displayed in Fig. 1, appeared yellowish-white in color and had a roughly spherical form with an approximate diameter of 2 mm. The biocarrier beads alsoinsoluble and mechanically stable in water. The particle density of a biocarrier bead was measured to be 0.288 g/cm3, which may be undesirable for application in column reactors because of the beads tendency to float in water. The porosity and the hydraulic conductivity of the beads were determined to be 0.370 and 1.455 cm/sec, respectively. Fig. 2(a) shows the scanning electron micrograph (SEM, S-2700; Hitachi, Tokyo, Japan) image of the surface of a resultant polysulfone biocarrier bead. The magnified
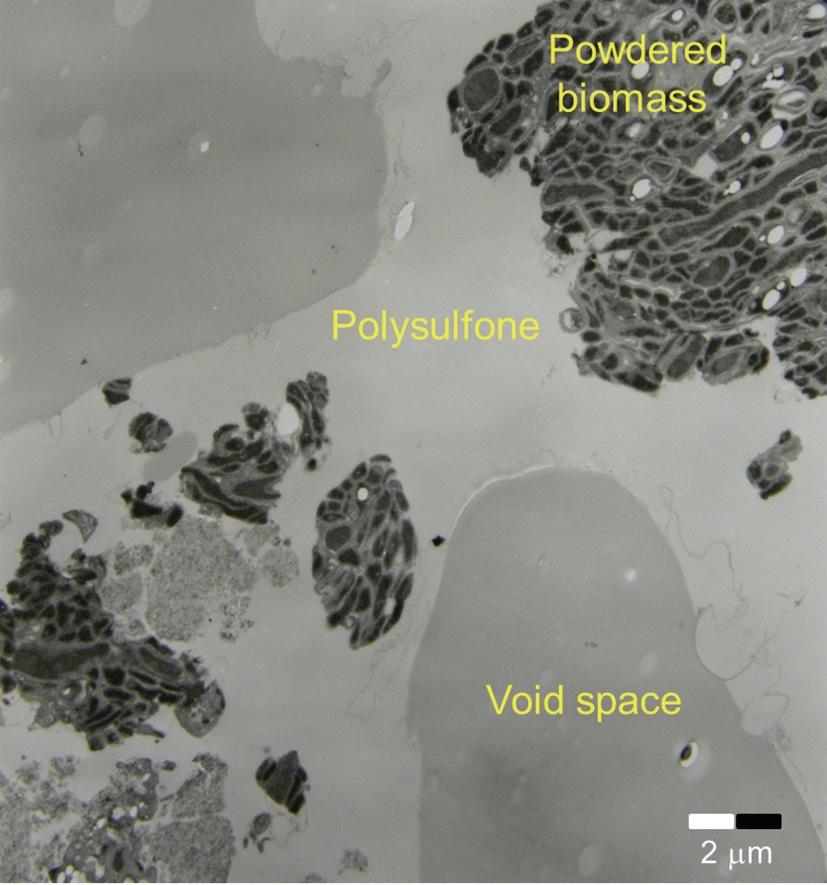
image (×70) revealed that the bead surface appeared irregular with some rough-spots scattered over the surface. No clear pattern for biomass immobilization was observed. The SEM image of the cross-section of the biocarrier in Fig. 2(b) clearly shows that the bead had a highly porous structure which is beneficial for the adsorption of metal ions. A thin skin-type outer layer was observed to cover the irregularly shaped pore structure, and the inner-most layer was composed of relatively large pore space and walls. The creation of the characteristic inside of the beads can be attributed to the precipitation of polysulfone and the diffusive flux of DMF solvent oozing out of the beads while they were rinsed [13]. Due to the highly porous structure, the specific surface area and pore volume of the biocarrier beads were measured by Brunauer-Emmer-Teller (BET) analysis to be 2.65 m2/g, which is 250 times larger than the external surface area, and 0.0067 cm3/g. However, it was difficult to visually distinguish polysulfone and biomass because of similar organic properties in the SEM images. In order to distinctively visualize the organic matters on the beads, transmission electron micrograph (TEM) (JEM-1210EX II; JEOL Ltd., Tokyo, Japan) analysis was also conducted. In Fig. 3, the TEM produced detailed surface images for the internal structure of a biocarrier bead at a magnification of 2,500. It revealed that the powdered debris of inactive
2.2. Preparation of Trace Metal Solution
1,000 mg/L stock solutions of lead and copper were prepared by using commercial standard solutions (AnApex Ltd., Daejeon, Korea). All working solutions for each batch experiment at a specified concentration were prepared from the stock solutions by successive dilution with distilled water. The pH of the solution was carefully monitored with a pH meter and was adjusted with dilute HNO3 and NaOH.
For the thermodynamic modeling and equilibrium isotherm fitting analyses, a series of batch experiments were performed by adding 2 g of biocarrier beads with 5% biomass content into 50 mL of metal solution at specified aqueous concentrations, which was established as optimized conditions in preliminary experiments. After approximately 24 hr of agitation on a rotary shaker at 120 rpm, the solution was separated from the biocarriers by a filter paper (Whatman No. 40, retention 8 μm; Whatman, Maidstone, Kent, UK) and the filtrates were analyzed by inductively coupled plasma-optical emission spectrometry (ICP/OES) (Optima 7000DV; PerkinElmer, Waltham, MA, USA) for the residual metal ions in the solutions. Various initial concentrations of Pb(II) and Cu(II) (0.01 to 100 mg/L) and different temperature conditions (from 293 to 313 K) were applied for the batch experiments. For kinetic analysis, biosorption batch experiments for Pb(II) and Cu(II), were performed with 2 g of biocarrier beads in 50 mL solutions with initial concentrations of 10 mg/L. These experiments were repeated for different contact times: 5, 10, 15, 20, 25, 30, 60, 180, 300, 480, 720, and 1,440 min. Solution samples were taken following the prescheduled sampling scheme for the residual metal concentration in the solution.
The amount of metal adsorbed at equilibrium,
where
All the batch experiments were conducted in duplicate and the mathematical mean values of two replicate measurements were applied to further analyses.
3.1. Biosorption of Metals by Bacillus drentensis Immobilized in Biocarrier Beads
To identify the change in the structure after biosorption experiments for Pb(II), the surface and the inside of a biocarrier bead were analyzed using SEM coupled with energy dispersive spectroscopy (EDS, EX-250; Horiba Scientific, Kyoto, Japan). Fig. 4(a) and (b) are the SEM images (×10,000) of the bead surface and the internal pore wall from a cutting cross-section, respectively. Tiny plaque-type solid crystals with a size of less than 1 μm were scattered on the bead surface and the inside pore wall. The EDS analysis focused on the crystals confirmed that this phenomenon resulted from adsorption of Pb(II) in a solid state. The distinct Pb peaks in the EDS spectra of the cluster on the surface and inside of the biocarrier bead were observed after the experiments. The porous structure of the biocarrier bead appeared to increase its sorption capacity by uptaking metal ions diffused
into the inside of the bead through an abundance of tiny pores on the surface. The SEM-EDS analysis, along with the decrease in Pb(II) concentration in the aqueous phase, confirmed that Pb(II) was successfully removed from the aqueous solution. Pb(II) was adsorbed as a form of crystal in the solid phase on the inside and the outside of immobilized biomass biocarrier beads.
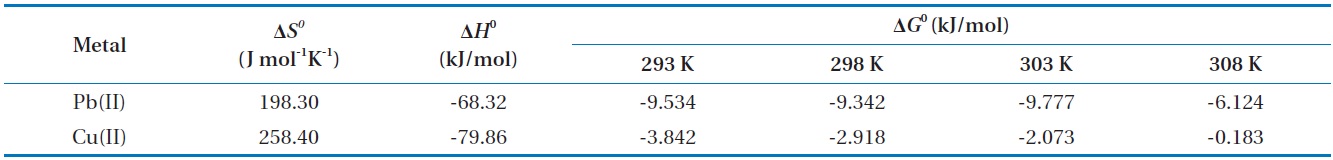
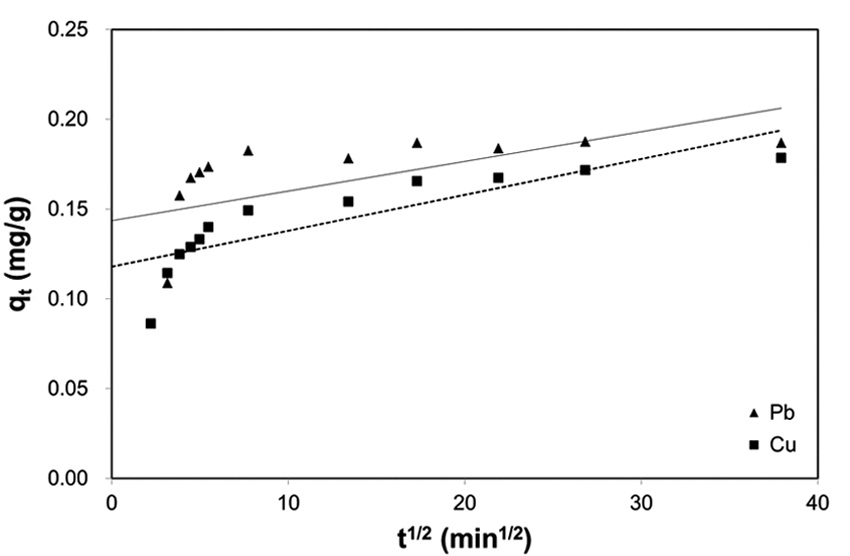
Thermodynamic analysis on the results from sorption experiments provides information on the nature of the sorption process, such as spontaneity or feasibility, which are important in engineering practice. The thermodynamic parameters such as Gibbs free energy change (Δ
where
Thermodynamic parameters for biosorption of Pb(II) and Cu(II) by immobilized biomass biocarrier beads
between Δ
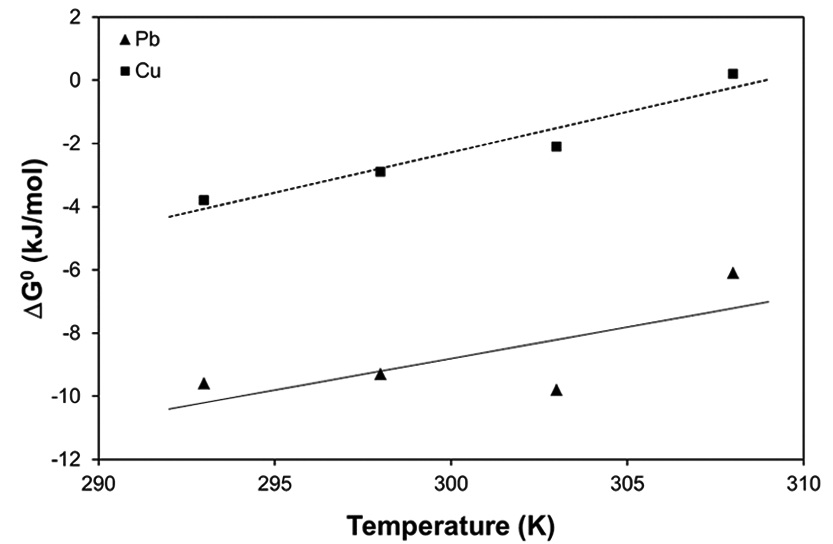
The Gibbs free energy changes (Δ
and gradually increased with the increase in temperature. The negative Δ
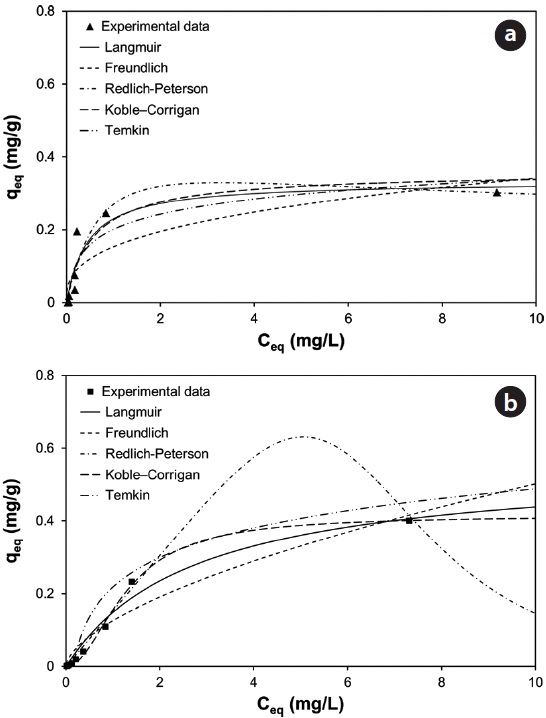
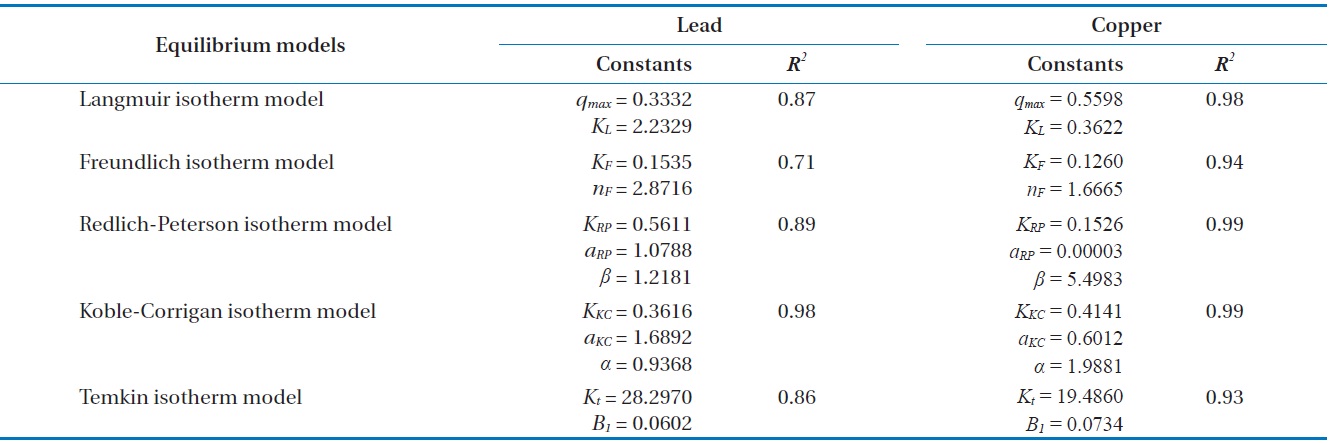
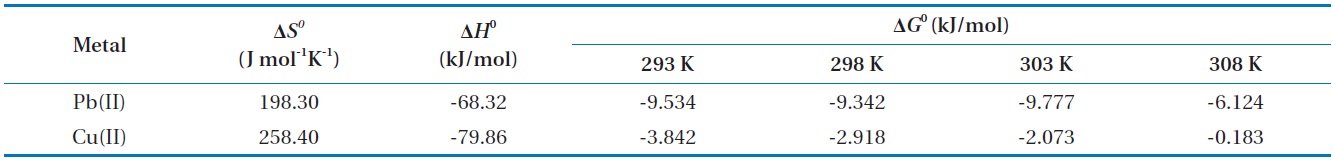
With underlying physical, chemical, or mathematical assumptions, the adsorption isotherms can provide information on biosorption mechanisms and surface properties and affinity of the biosorbents. In this study, a series of biosorption batch experiments for 2 g of biocarrier beads at 20℃ and different metal concentrations (from 0.01 mg/L to 100 mg/L) were conducted and the equilibrium distribution of Pb(II) and Cu(II) was respectively plotted in Fig. 6(a) and (b). It was observed that the amount of metal adsorbed at equilibrium by the unit mass of biocarriers (
For further analyses, the results were fitted by five widely applied adsorption isotherms including Langmuir, Freundlich, Redlich-Peterson, Koble-Corrigan and Temkin isotherm models as follows.
where
Fig. 6(a) and (b) show the amount of Pb(II) and Cu(II) adsorbed onto the unit mass of biocarrier beads at equilibrium at different initial concentrations and adsorption isotherms fitted to the experimental data. The sorption increased as the equilibrium concentration of Pb(II) and Cu(II) in the aqueous phase increased. The comparison of the correlation coefficients in Table 2 confirmed that the equilibrium biosorption data of Pb(II) and Cu(II) fit very well to the Koble-Corrigan, Redlich-Peterson and Langmuir isotherms compared to the Freundlich or Temkin isotherm. Despite the mathematically good fit and high correlation with experimental data, the Redlich-Peterson isotherm, which was originally suggested as a combined form of Langmuir and Freundlich isotherms, was not found suitable to describe the biosorption of Cu(II) because of its innate constraint of 0 <
Adsorption isotherm parameters for biosorption of Pb(II) and Cu(II) by immobilized biomass biocarrier beads
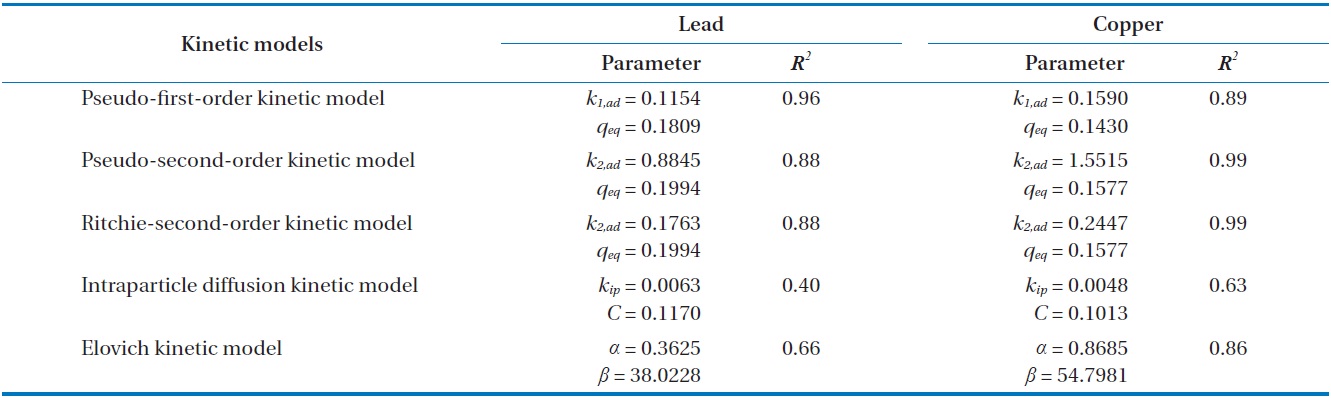
Kinetic parameters for metal biosorption of Pb(II) and Cu(II) by immobilized biomass biocarrier beads
where
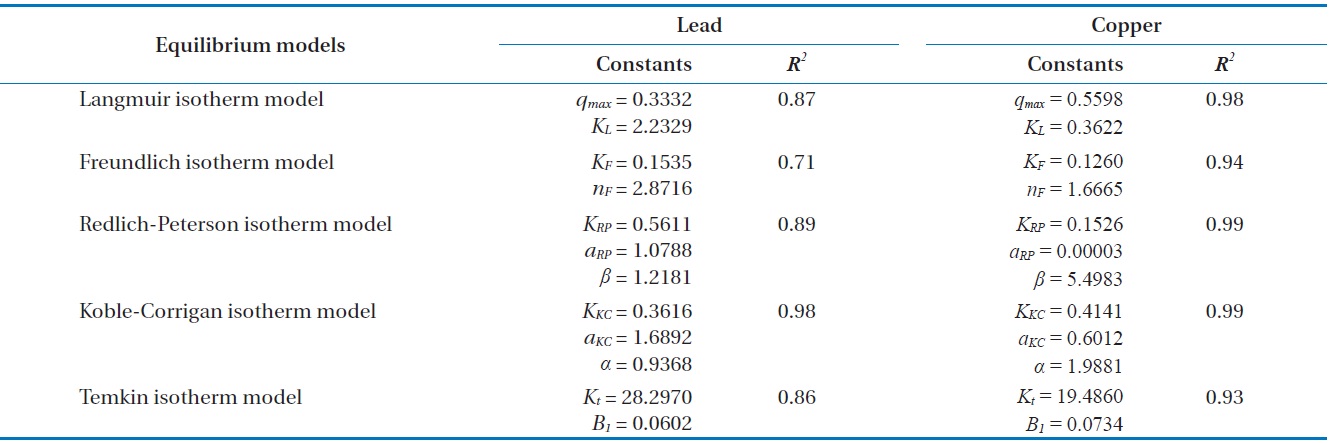
Results from batch experiments were fitted by five widely applied kinetic models including pseudo-first-order, pseudo-second- order, Ritchie second-order, intraparticle diffusion, and the Elovich kinetic model. The nonlinear forms of the tested kinetic models are as follows.
where
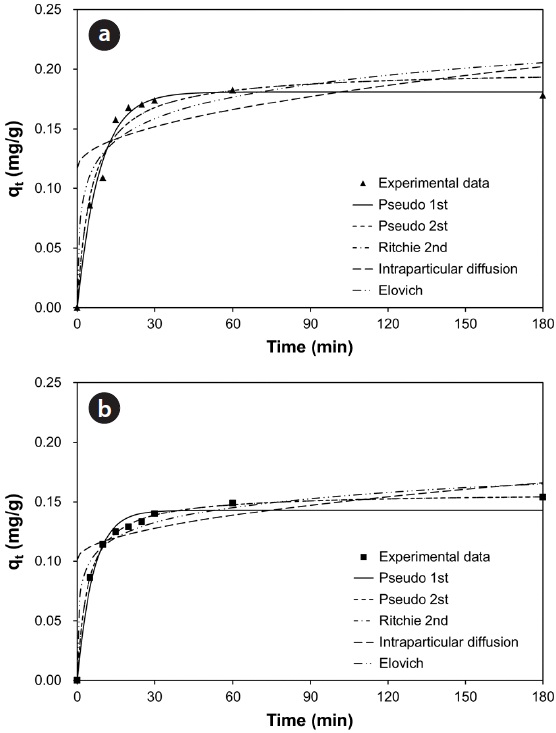
Fig. 7(a) and (b) show the changes in the amount of adsorbed metal ion (
model, 96.7% and 95.7% of total Pb(II) and Cu(II) biosorption were accomplished within the initial 30 and 60 min, respectively. As the saturation or equilibrium state was approached, the biosorption proceeded slowly and became ineffective. In this later stage, the remaining vacant sorption sites on the surface were competitively occupied by the sorbates and intraparticle biosorption gradually continued as the metal ions were diffusing into the interior of the biosorbents. The total contact time of 40 min for Pb(II) and 124 min for Cu(II) were estimated to be required to reach near equilibrium (99% of maximum biosorption).
Comparing the correlation coefficients for the applied kinetic models to experimental data, the pseudo-first-order kinetic produced the best fit for Pb(II), while the pseudo-second-order and the Ritchie second-order kinetics produced the best fit for Cu(II). The second-order kinetics and the Elovich kinetic also fit the experimental data for Pb(II) and Cu(II) relatively well, respectively. In these nonlinear regressive calculations, the pseudo-secondorder and the Ritchie second-order kinetics generated almost identical fits. The intraparticle diffusion kinetic could not represent biosorption of both Pb(II) and Cu(II) by the biocarrier beads. In general, first-order kinetic models are reported to be applicable to the initial stage of a sorption process, but not over the entire range of adsorption processes [26]. However in this study, the pseudo-first-order kinetic model generated the best representation for biosorption of Pb(II) with the process proceeding in a very short time. In less than 1 hr, equilibrium had been reached and no further changes occurred. Overall, the biosorption of Pb(II) and Cu(II) by immobilized biomass biocarrier beads could be represented by the pseudo-second-order and Ritchie secondorder kinetics over the entire biosorption process.
Biosorption by granular porous sorbents is a multi-step process which consists of bulk diffusion in bulk liquid, external film diffusion between the bulk liquid and sorbent surface, intraparticle diffusion inside a particular sorbent, and interaction between sorbates and sorption sites over the interior pore structure [27]. In order to investigate the contribution of intraparticle diffusion in the multi-step sorption mechanism, the intraparticle kinetic model proposed by Weber and Morris [24] have been widely applied [23,25,28]. According to the Weber-Morris model, the plot of experimental data for
In this study, the dead biomass (