



Until now, various process technologies for NDMA treatment in water have been proposed. Previous studies have evidently shown that UV treatment is the most effective method due to the strong photo-sensitive NDMA [8,10,15-18]. Furthermore, the photo-mechanism for the decomposition of NDMA during UV-based treatment has extensively been studied [6,8,15,16,19]. However, in the UV photolysis there are concerns of nitrosamines reformation including NDMA occurrence after UV irradiation [8,11,20]. Thus, another approach to prevent the reformation of NDMA is necessary.
As an alternative method, the photodecomposition of NDMA has been performed in the presence of H2O2 [10]. The UV/H2O2 process utilizes further oxidation of NDMA by itself and/or NDMA degradation byproducts such as dimethylamine and nitrite as representative NDMA precursors via the hydroxyl radical (?OH). In this process, H2O2 as a source of hydroxyl radical may be quite useful for controlling NDMA reformation in post-UV treatment [8,10,20]. According to Sharpless and Linden [10], the addition of H2O2 in the photo-degradation of NDMA slightly enhanced the performance by using a low pressure Hg lamp, but economic benefit in view of practical engineering effectiveness was little or none. Since their study, however, additional study on the photodecomposition of NDMA in the presence of H2O2 has not been continued. Furthermore, new reactive species and a new mechanism that can be generated from the addition of H2O2 during the photodecomposition of NDMA have not been reported recently, to the best of our knowledge. In particular, the chemistry of reactive species generated from the direct photolysis of NDMA is of considerable significance in water purification research for those mechanistic roles and an important accompanying role of the secondary product [19]. This information is
able to contribute to the development of the kinetic computer model for the well-defined or proposed chemistry in the system that provides the best test of the actual engineering data. In this point, a critical component for the kinetic modeling is a description of the kinetics and mechanisms of the reactions for all the chemical species involved in the decay of NDMA. Thus, an understanding of the reaction mechanisms that could take place in the presence of H2O2 is necessary in NDMA photodecomposition.
The objective of this study is to experimentally reveal the formation of unknown reactive species (URS) and its reactivity toward NDMA photo-degradation in the presence of H2O2. To investigate the formation of URS, this study employed mechanistic probing compounds which are
BA, methanol, NDMA, and 30% H2O2 (Sigma-Aldrich, St. Louis, MO, USA) were reagent grade and were used as received from commercial supplies; water was purified with use of an aquaMAX model (Young Lin, Anyang, Korea). The PNDA (99%; Acros Organics, Geel, Belgium) was also used without further purification. To examine competition and subsequent NDMA reactions, all PNDA solutions were prepared by the addition of small amounts of borate buffer solution (pH = 8.75; LabChem, Pittsburgh, PA, USA). The concentration of H2O2 stock solution was determined with the KMnO4 titration method prior to use. The working solution of H2O2 was prepared daily by diluting the stock solution with water.
2.2. Experimental Apparatus and Procedures
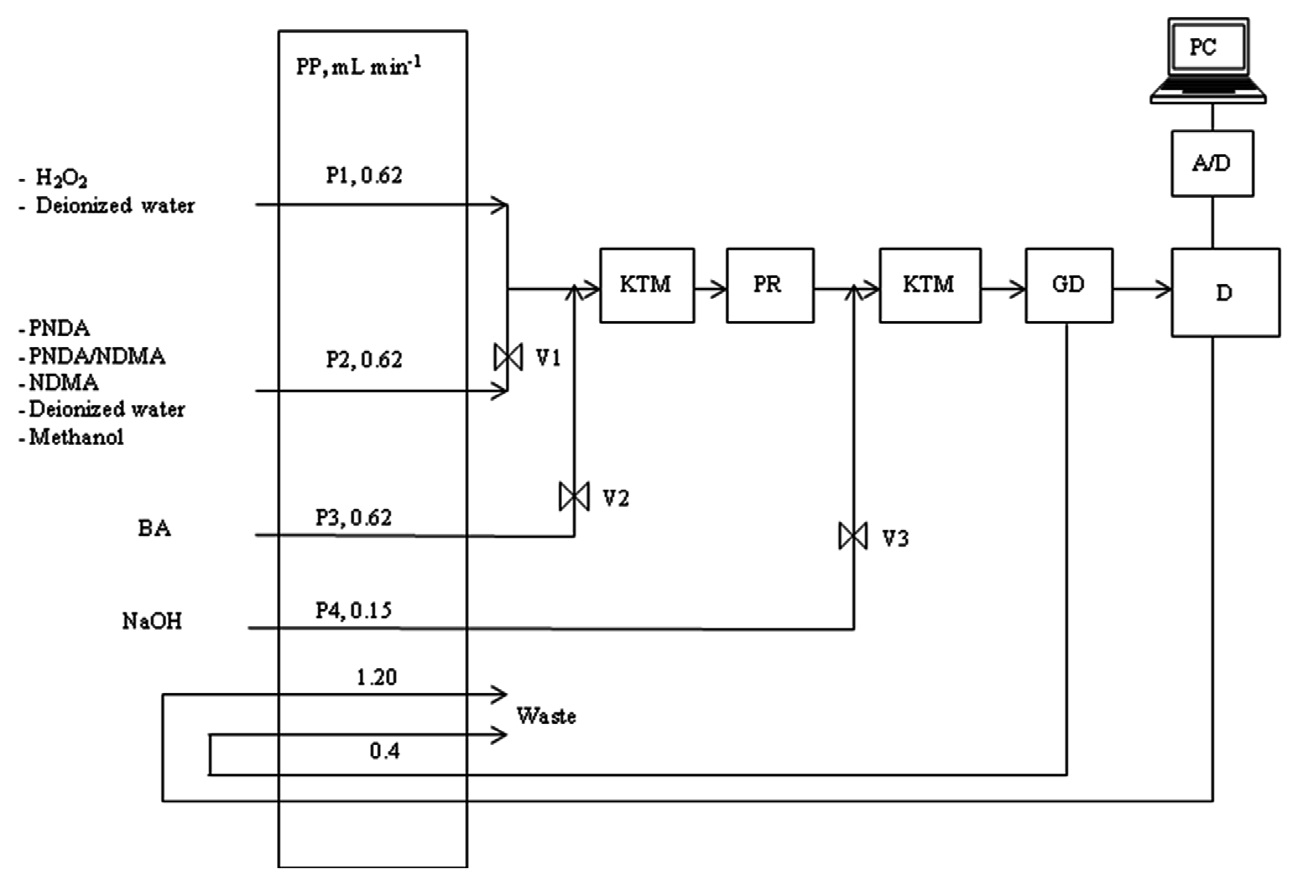
Fig. 1 shows a schematic diagram for the experimental apparatus used in this study. The apparatus and experimental procedures employed in the present study are similar to a previous study [19] except for the port of H2O2 solution. All solutions were delivered with a peristaltic pump (PP; BVP-Process IP65 model; Ismatec, Glattbrugg, Switzerland) with polytetrafluoroethylene (PTFE) tubing (i.d. 0.8 mm; Cole-Parmer, Vernon Hills, IL, USA).
As shown in Fig. 1, water or the H2O2 solution was delivered through port 1 (P1, 0.62 mL/min). At the same time, PNDA solution alone or a mixed solution of NDMA and PNDA or methanol was delivered through port 2 (P2, 0.62 mL/min), which joined with the H2O2 solution or water. A 1 m long knotted tubing mixer (KTM) [23] premixed the solutions of PNDA and NDMA, PNDA only, or a mixture of NDMA/PNDA/H2O2. During a competition reaction of PNDA and NDMA on ?OH and/or URS, valve 1 (V1) was opened, while valve 2 (V2) and valve 3 (V3) were closed. In the meantime, during the detection of
Then, the premixed solutions were transferred to a quartz coil-type photo-reactor (PR), and these solutions inside the quartz coil-type PR were photolyzed by irradiation from a low pressure Hg lamp (4 W, λmax = 254 nm; Sankyo Denki G4T5, Kanagawa, Japan). The lamp was placed at the inner center of the PR. In this PR, H2O2 is cleaved by absorbed light, producing ?OH. After mixing in the second KTM, the photolyzed solution containing decayed NDMA and/or PNDA was delivered into a homemade glass de-bubbler (GD), which was placed prior to the detector to remove formed bubbles. The changes in the absorbance signal of PNDA and the fluorescence signal of OHBAs were monitored for reaction times by a UV/Vis spectrophotometer (730D model; Young Lin) and by a fluorometer (474 model; Waters, Milford, MA, USA), respectively. Finally, the detected signal was transferred to a data acquisition system, which consisted of a signal amplifier, analog-to-digital converter, and personal computer.
Additionally, the induction period for photolysis (or competition reaction) was approximately 40 sec, which was based on the absorbance signal, with the total irradiation time less than approximately 3 min. To analyze NDMA, irradiated samples were repeatedly collected into sample vials at constant time intervals throughout repeated experiments.
2.3. Competition Reaction of PNDA and NDMA for a URS
A competition reaction was used to identify a URS having ·OH-like reactivity. In this process, ?OH is produced during the decomposition of H2O2 (reaction 1). ?OH reacts with PNDA (reaction 2,
The competition kinetic model for the NDMA and PNDA in this study is Equation 1 (E1) listed below:
where So represents the loss rate of PNDA in the absence of NDMA, and S represents the loss rate of PNDA in the presence of NDMA; [PNDA]0 and [NDMA]0 in E1 are the initial concentrations. Control experiments consisted of the same mixture and irradiation conditions without the H2O2. Since
NDMA was analyzed by high performance liquid chromatography (2690 Model and PDA 996; Waters) with a μBondapak C18 (3.9 × 300 mm; Waters) employing UV detection at 228 nm. The eluent consisted of water:phosphoric acid = 99:1 (volume %) with a flow rate of 0.8 mL/min [19]. The detection limits of this method was approximately 0.1 μM, based on the signal-to-noise ratio (SNR) = 3.
All absorbance measurements of PNDA were adjusted with borate buffer solution because the maximum extinction coefficient, 32,282/M/cm, of PNDA at 446 nm was observed at pH 8.75 [26]. The concentration of PNDA was determined in a continuous
flow by using a UV/Vis spectrophotometer (730D; Young Lin).
To identify the formation possibility of URS and/or ?OH driven from NDMA photodecomposition with H2O2, OHBAs as main products of BA trapped ?OH were determined with a fluorescence detector (474 model; Waters) at 400 nm (emission), which was excited by 320 nm (excitation) [23]. A 0.5 N NaOH solution (pH ≥ 11) was used to enhance fluorescence intensity, as shown in Fig. 1.
The dissolved oxygen (DO) was measured with a DO meter (SensorLink PCM800; Thermo Scientific Orion, Waltham, MA, USA).
3.1. NDMA Photodecomposition by Reactive Species in the Presence of H2O2
To account for the dependences of reactive species (or URS) and the UV photolysis on NDMA decay in the presence of H2O2, a series of experiments were carried out over a range of initial NDMA concentrations (6.25?50 μM) at pH 8.75. In each experiment, the relative importance on H2O2 effect during NDMA photolysis was investigated, based on the initial rate (r = -
Fig. 2 shows r/rmax as a function of [H2O2]o/[NDMA]o in a given NDMA concentration at pH 8.75. As shown in Fig. 2, r/rmax was gradually increased with increasing molar ratio of [H2O2]o/ [NDMA]o, and then it was constant at the molar ratio of [H2O2]o/[NDMA]o = 80?100. In particular, the ratio of r/rmax without H2O2 at a given NDMA concentration was 0.65, and its ratio at a molar ratio of [H2O2]o/[NDMA]o = 80?100 was approximately 1, as shown in Fig. 2. This result suggests that the ratio of r/rmax
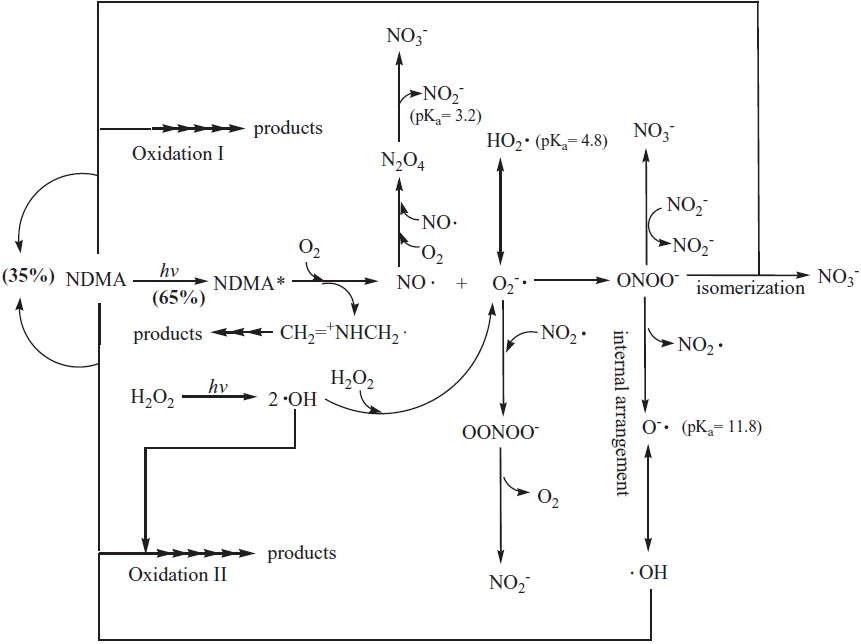
is independent of the initial NDMA concentration, but this ratio at above 0.65 is dependent on the initial H2O2 concentration added. In other words, as described in Fig. 3 approximately 65% of NDMA decay was accomplished by the direct photodecomposition of NDMA by UV light, whereas about 35% referred to the effect of reactive species (i.e., ?OH) and/or URS generated through the photolysis of H2O2 (reactions 1 and 5) and a subsequent unknown reaction(s). This result seems to be similar to that observed by Sharpless and Linden [10]. According to their study, the addition of 2.94 mM H2O2 in the photo-degradation of NDMA lead totely a 30% increase in performance by the low pressure Hg lamp, compared with treatment using UV alone in the absence of H2O2. Even though the initial rate of NDMA photo-decay was mostly explained by UV photolysis, the addition of H2O2 considerably enhanced direct photolysis of NDMA. This result can be explained by considering the photochemically generated ?OH and/or URS. Thus, the effect of H2O2 can be quantitatively explained by the reactive species including URS.
3.2. Competition Reaction between Probes and NDMA for URS
In this study, a competition reaction was used to identify the reactivity of an URS or reactive species. Reactive species including URS can be produced through NDMA photo-decay in the presence of H2O2, in which a hydroxyl radical (reaction 1) and URS (reaction 5) are able to competitively react with PNDA and NDMA [19]. Hence, the competition reaction between NDMA and PNDA as a well-known representative probe for ?OH on an URS was investigated in this study. In this experiment, the concentrations of H2O2 and PNDA were always constant with 10 and 2 μM, respectively, at pH 8.75. As a result, NDMA concentrations
were varied in a wide range from 0 to 40 μM depending on the molar ratio of [NDMA]o/[PNDA]o.
Fig. 4 presents So/S as a function of [NDMA]o/[PNDA]o. As shown in Fig. 4, So/S was slightly increased into a molar ratio = 1 of [NDMA]o/[PNDA]o, and thereafter, these values were rapidly decreased with an increasing ratio of [NDMA]o/[PNDA]o. At the molar ratio = 20 of [NDMA]o/[PNDA]o, So/S was 0.62. This result shows that the S of URS generated in the presence of NDMA is much higher with approximately 1.61 times than So in the absence of NDMA. In other words, the decomposition rate of PNDA was dependent on the initial concentration of NDMA, which
means a marked increasing PNDA decay rate with increasing initial concentrations of NDMA. This result was unexpected in the competition kinetics under the same condition with 10 μM H2O2, even though NDMA was strongly photo-labile.
In the general competition kinetics, S in the presence of NDMA should be smaller than So in the absence of NDMA, and thus So/S vs. [NDMA]o/[PNDA]o generally produces a linear slope of the rate constant ratio (
To further examine a URS having ?OH-like reactivity during the photolysis of NDMA, a series of experiments were performed in the presence of 1 mM BA as a ?OH probe and in a range of initial H2O2 concentrations (10?1,000 μM). BA is a well-known ?OH probe (
Fig. 5 demonstrates the formation of OHBAs in the photodecay of NDMA. As shown in Fig. 5, OHBAs produced through reactions between ?OH and BA were linearly increased with increasing initial concentration of NDMA at a given concentration of H2O2. This indicates that URS having ?OH-like reactivity and/or additional ?OH could be produced by an unknown pathway during the photochemical decay of NDMA, influencing intermediates or other products. Thus, a URS having ?OH-like reactivity can influence the photolysis of NDMA.
To further examine URS formed during the photolysis of NDMA, methanol was used as an alternative probe for ?OH, which is able to scavenge ?OH [29-31]. A series of experiments were performed in the presence of H2O2 and at pH 8.75. At this time, the concentration of methanol added was varied in excess ranging from 0 to 50 mM, compared to the concentration of NDMA.
As shown in Fig. 6, an excess of methanol (50 mM) did not interfere with the photo-decay of NDMA, but, rather, methanol of 30 mM or less improved the photo-degradation of NDMA slightly. Even though ?OH would be produced in this experiment, most of ?OH is able to be scavenged by of methanol, producing a hydroperoxyl radical (HO2?) and HCHO as final products (reactions 6?8) [29]. This result suggests that an URS formed during the photodecomposition of NDMA reacts with NDMA. As a result, the slightly enhanced decomposition of NDMA could result from a URS formed by the photolytic behaviors of NDMA in the presence of methanol.
3.3. Possible Mechanism of URS formation in the Photodecomposition of NDMA
As shown in Figs. 2?5, a URS generated from the course of the photo-degradation of NDMA in the presence of H2O2 would be a strong reactive species having ?OH-like reactivity. To the best of our knowledge, one of the plausible explanations on the URS is additional ?OH and/or peroxynitrite (ONOO-), as described in Fig. 3.
Excited NDMA under UV light irradiation releases NO? by photo-elimination from the homolytic cleavage of N-NO bonds (reactions 9?10), and by photo-hydrolysis from the heterolytic cleavage of N-NO bonds (reaction 11) [6,16,17]. Here, NDMA is photolyzed to dimethylamine (CH3 = NHCH3) and the aminium radical (CH2 = +NHCH3?) which have very weak reactivity [6]. In the initial step, NO? and the superoxide anion radical (O2 -?) in an excess of oxygen (DO = 0.39 mM) could undergo a rapid combination reaction to yield the powerful oxidant ONOO- (reaction 12). The generation of NO? and the concomitant formation of O2 -? were confirmed by Chow [6] and by Lee et al. [16], respectively. Thus, URS as a new reactive species can be related to the formation of ONOO-.
In the meantime, HO2?/O2 -? formed from reaction 3 and reaction 11 is dependent upon the acid-base equilibrium (reaction 14) [32]. Considering our experimental conditions (pH = 8.75), self-disproportionation by reactions 15?16 (
Recent studies have revealed that the decomposition of ONOO- can generate NO2? and ?OH with a 30% yield as well as NO3- with a 70% yield [34], even though the reactivity of ONOO- and its decay pathways have been in dispute [34-39]. In addition, according to Koppenol et al. [36], ONOO- can be a strongly oxidizing compound by itself. Hence, the reactivity of ONOO- is closely intertwined with that of ?OH, and appears to have significant rivaling to that of ?OH.
As shown in Figs. 3?5, at the alkaline pH solution, both loss of PNDA and formation of OHBAs providing experimental evidences in support of URS and/or ?OH formation were dependent on NDMA concentrations during the photolysis with the constant concentration of a given H2O2. In particular, as shown in Fig. 6, an excess of methanol (≤ 30 mM) improved the photodecomposition of NDMA slightly. These results suggest that a highly reactive oxidizing species can be ONOO- by itself or ?OH. Therefore, our data suggest that, presumably, a highly oxidizing URS is formed in the photolysis of NDMA in the presence of H2O2, which is ONOO- as a potent oxidant, as described in Fig. 3.
Careful work in this study led to the discovery that a URS generated from NDMA photolysis with a constant concentration of H2O2 added was enhanced with increasing NDMA. The detailed study was performed with competition kinetics and probes for URS. Both loss of PNDA and formation of OHBAs were dependent on concentrations of NDMA during the photolysis of NDMA with 10 μM H2O2 added. In particular, the competition kinetic method demonstrated that the relative reactivity of a URS was identical with ?OH-like reactivity. These results suggest that a highly oxidizing URS is ONOO- as a potent oxidant by itself as well as a source of ?OH.

![Normalized initial rates (r/rmax) of N-nitrosodimethylamine (NDMA) photodecomposition plotted against [H2O2]o/[NDMA]o. Experimental condition: pH, 8.75; and wavelength, 254 nm.](http://oak.go.kr/repository/journal/12049/E1HGBK_2013_v18n1_29_f002.jpg)
![So/S plotted against [NDMA]/[PNDA]. Experimental conditions: pH, 8.75; [H2O2]o = 10 μM, [PNDA]o = 2 μM; and wavelength, 254 nm. Note that the dashed line is a linear line generally expected in the competition kinetics. NDMA: N-nitrosodimethylamine, PNDA: p-nitrosodimethylaniline.](http://oak.go.kr/repository/journal/12049/E1HGBK_2013_v18n1_29_f004.jpg)
![Fluorescence signal intensity of OHBAs from BA depending on initial NDMA concentration added in the photolysis of NDMA. Experimental conditions: pH, 8.75; [BA]o = 1 mM; and wavelength, 254 nm. Note that the signal intensity of OHBAs (arbitrary unit), which means the fluorescence signal intensity produced from URS during the photo-degradation of NDMA, subtracts the fluorescence signal intensity of H2O2 alone from the total fluorescence signal intensity in the presence of NDMA and H2O2. NDMA: N-nitrosodimethylamine, OHBA: hydroxybenzoic acid, BA: benzoic acid, URS: unknown reactive species.](http://oak.go.kr/repository/journal/12049/E1HGBK_2013_v18n1_29_f005.jpg)
![N-nitrosodimethylamine (NDMA) photodecomposition depending on methanol added in the photolysis of NDMA. Experimental conditions: pH, 8.75; [H2O2]o = 10 μM; and wavelength, 254 nm.](http://oak.go.kr/repository/journal/12049/E1HGBK_2013_v18n1_29_f006.jpg)