EE2 (17α-ethinylestradiol) is an orally bioactive estrogen, which has been widely used in both human medications and livestock management activities. This synthetic hormone has become a widespread contaminant in the aquatic environment, mainly because of its high resistance to degradation, and its tendency to interfere with and/or deregulate the endocrine system of living organisms in contaminated environments (Aris et al., 2014). A number of previous studies have reported the detrimental effects of EE2 on aquatic organisms with particular emphasis on damage to the reproductive systems of bony fishes. These include the changing of the sex ratio and reproductive capability (Lange et al., 2001), changed gene expression profile related to sexual maturation (Filby et al., 2007; Hirakawa et al., 2012), and alteration of male competitive courtship behavior (Lee et al., 2014). The adverse effects of EE2 on fish have not been limited to reproductive issues. EE2 has also been reported to alter the growth and differentiation patterns, innate immunity, apoptosis inhibition, and metabolomic profiles in exposed fish (Shved et al., 2008, 2009; Nadzialek et al., 2010; Teng et al., 2013). For these reasons, the development of biomarker-assisted approaches to elucidate the mechanism (s) underlying the influence of EE2 on the cellular and physiological pathways in fish is crucial to address the effects of EE2 on contaminated biota (Filby et al., 2007; Harding et al., 2013).
A number of transgenic fish models incorporating fluorescence protein reporters have been established for detecting waterborne estrogenic compounds, in which estrogen-responsive promoters were designed to drive the expression of visible fluorescent protein in specific response to exposure to exogenous estrogenic chemicals (Chen et al., 2010; Petersen et al., 2013). As an
We recently reported the potential of a transgenic system constituting a fluorescent reporter gene driven by a choriogenin H promoter developed in a euryhaline marine medaka (
The transgenic marine medaka individuals used in the present study were F4 generation transgenic individuals carrying the red fluorescent protein gene (
>
Laboratory breeding and preparation of larval samples for exposure
To obtain hemizygous transgenic larvae for the exposure studies, group mating was carried out between five-month-old transgenic males (half-siblings;
>
EE2 exposure with different age groups of transgenic hatchlings
To examine the effects of the initial treatment duration on the success of transgene induction, hatchlings collected at 0, 1, 2, 3, 6, and 9 days post-hatching (DPH) were exposed to 500 ng/L (nominal concentration) of EE2 for 3 days. The EE2 used in this study was analytical grade reagent purchased from a commercial supplier (Sigma-Aldrich, St Louis, MO, USA). For exposure, larvae (
>
Experimental exposure to high concentrations of EE2
Due to the age-dependence of the frequency of transgene induction, transgenic hatchlings of the same age, 7 days, were exposed to various concentrations of EE2 up to 1,000 ng/L. The larvae (approximately 40 fish per group) were exposed to 0 (non-exposed), 200, 400, 600, 800 or 1,000 ng/mL EE2 for 3 and 6 days. All tank conditions including temperature and salinity were the same as those described above. During the exposure period, 50% of the water was exchanged daily and the hormone was simultaneously replaced with fresh hormone. As in the procedure described above, PCR screening of the transgene was conducted with RFP-negative fish to estimate the frequency of transgene expression. Duplicate exposure tests were carried out.
>
Experimental exposure to low concentrations of EE2
To examine the potential for detecting waterborne EE2 of dose strengths close to environmentally relevant concentrations, transgenic larvae were exposed to low doses of EE2. Five or six-day-old transgenic larvae were exposed to EE2 at dose levels of 0 (non-exposed control), 25, 50, and 100 ng/L. Approximately 48 larvae per group were incubated in a 10-L rectangular tank containing 8-L 10-ppt water at 25℃. During exposure, the fish were fed a 100-μm powder diet for flounder as above and the daily exchange rate of hormone-supplemented water was 30%. From 24 h after exposure, the individual fish from each group were examined daily for the occurrence of RFP signals using fluorescence microscopy. At the end of the exposure period, the larvae were subjected to PCR transgene screening to estimate the efficiency of transgene induction in each group.
>
EE2 exposure under different salinity conditions
The effects of salinity on the EE2-mediated RFP expression were examined in the transgenic larvae. At 2 DPH, the hatchlings in the 10-ppt tank were acclimated to one of three salinities (0, 15 and 30 ppt) according to the procedures described in our previous work (Cho et al., 2010). After five days of acclimation at each salinity level, 7-day-old larvae (
>
Effects of larval age on the success of transgene expression during EE2 exposure
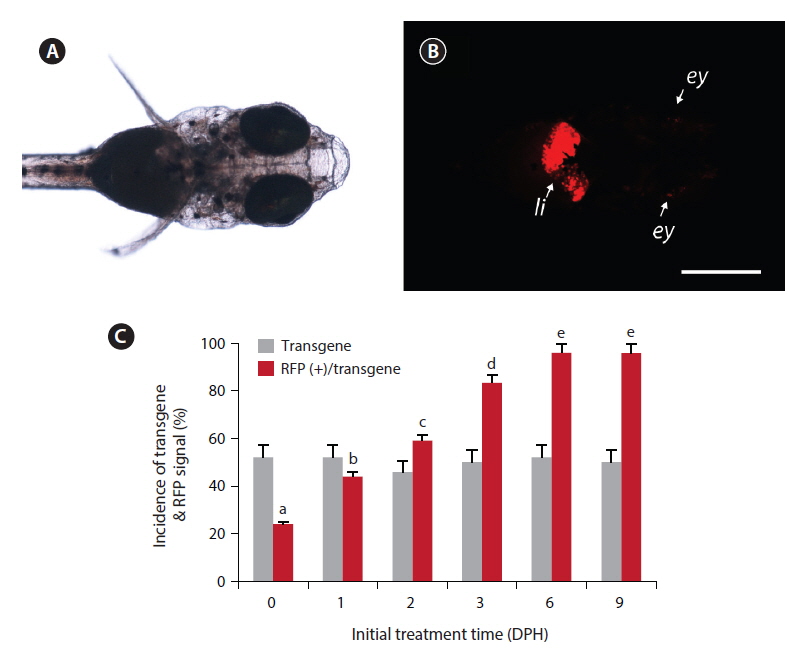
Under the microscopy conditions used in this study, none of non-transgenic larvae showed any notable red fluorescence signal, with the exception of minute background fluorescence around the eyes (not the lens). Also, no transgenic larvae displayed apparent RFP expression under non-exposed conditions, including the transgenic larvae in the ethanol-treated control groups. However, among the groups exposed to EE2, the transgenic larvae displayed a strong RFP signal in their livers (Fig. 1). The liver-specific induction of RFP signals was consistent in all the RFP-positive larvae, indicating that the liver-dominant regulation of choriogenin H promoter was well manifested in transgenic larvae in response to exogenously introduced EE2. However, in a previous study, it was reported that choriogenin H gene transcripts were detectable in several non-liver organs of adult marine medaka (Lee et al., 2012b), which differs from our findings of liver-exclusive appearance of RFP signals in transgenic larvae. Hence, further studies exploring the regulation mechanisms of the choriogenin H gene in non-liver tissues throughout the life cycle of marine medaka are necessary.
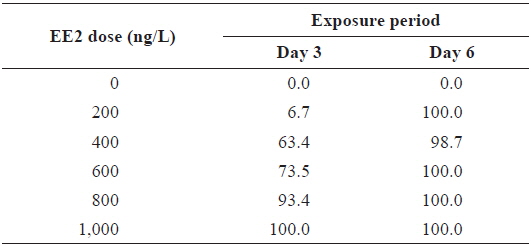
Although EE2-mediated induction of RFP was apparent in transgenic larvae, the frequency of RFP expression was significantly affected by the larval age (
>
Semi-quantitative expression of transgenic RFP during exposure using high concentrations of EE2
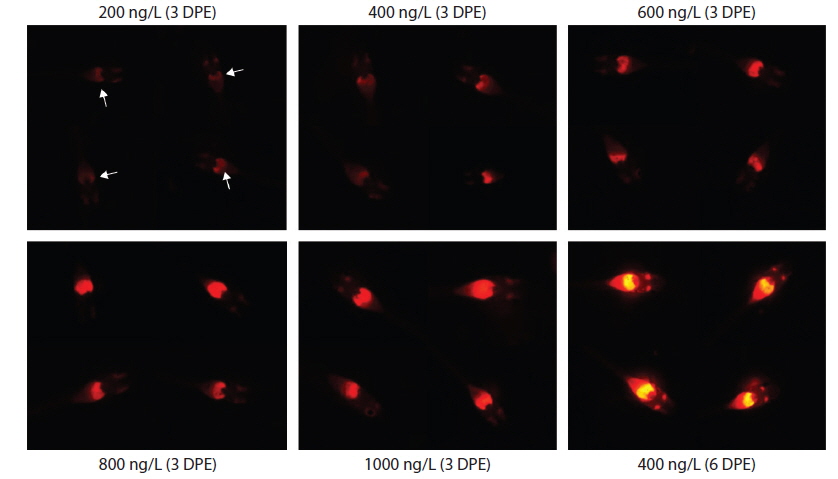
During EE2 exposure with concentrations ranging from 200 to 1000 ng/L, the frequency of RFP expression was, in general, positively related to EE2 concentration at 3 DPE (Table 1). The 200 ng/L-treated group showed less than 10% incidence of RFP expression, which increased to 63% at 400 ng/L, and further increased with higher EE2 doses to 90–100%. However, the observed dose dependency decreased at 6 DPE, for which most exposed groups showed 100% expression of RFP. The dose-dependent appearance of RFP-positive larvae at 3 DPE may reflect differential EE2 uptake by the fish during the early phase of exposure. On the other hand, the plateau of RFP induction at 6 DPE may be related to the rapid bioaccumulation of EE2 during the extended exposure period, which is consistent with previous reports that EE2 is highly resistant to biodegradation (Zhang et al., 2006; Aris et al., 2014). In addition to the induction efficiency, fluorescence microscopy analysis showed that the RFP intensity in the livers of the transgenic larvae was also positively correlated with EE2 dose, particularly at 3 DPE (Fig. 2). Under the assay conditions used in our study, the dose-dependent increase in RFP intensity in the livers of the transgenic larvae was apparent at 3 DPE, although there were also variations among individuals within a given exposure dose, suggesting that transgenic detection of EE2 could be performed, in at least a semi-quantitative manner (Cho et al., 2013). However, at 6 DPE, most, but not all, transgenic larvae showed a tendency toward saturation of fluorescence regardless of EE2 concentration. Hence, further studies are required to compare the actual tissue burden (
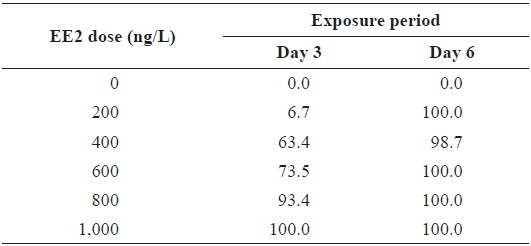
Incidence of RFP-positive transgenics during experimental exposures to different concentrations of EE2 (17α-ethinylestradiol) for 3 or 6 days*
>
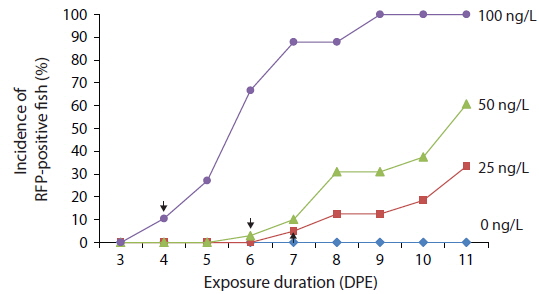
Time course expression patterns of transgenic RFP during exposure to low concentrations of EE2
Transgenic detection of lower concentrations of EE2 was also possible using
>
EE2-induced transgene expression under different salinity conditions
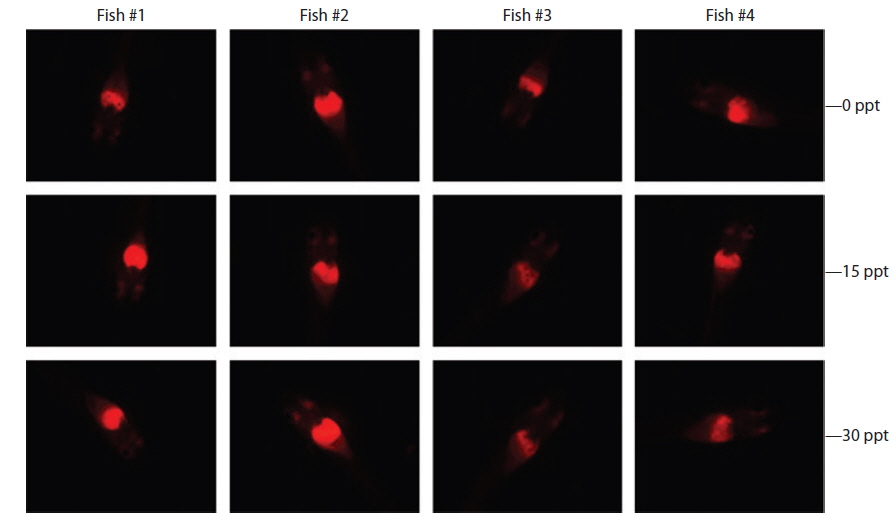
Under the exposure conditions used in the present study, transgenic expression at the phenotypic level in response to EE2 exposure did not differ among salinity groups in either the intensity or incidence of the RFP signal acquired in the transgenic larvae. After 1 week of exposure, all salinity groups (0, 15, and 30 ppt) displayed a high incidence of RFP induction (89.5% for 0 ppt, 91.3% for 15 ppt, and 89.5% for 30 ppt). The RFP intensity induced in the livers of the transgenic larvae assessed by fluorescence microscopy was also similar among all salinity groups, although some inter-individual variation was observed within each group (Fig. 4). These findings suggest that the
Our choriogenin H promoter-driven reporter transgenic system developed in euryhaline marine medaka represents a straightforward approach to the detection of various concentrations of waterborne EE2, a widespread estrogenic pollutant in aquatic environments. Based on its acceptable sensitivity, this transgenic approach using marine medaka offers significant potential for detecting EE2 in a semi-quantitative and environmentally relevant manner. To build upon this study, detailed investigation of both the biotic and abiotic factors affecting the sensitivity and efficiency of transgenic expression should be carried out.