It is widely known that anesthetics may disturb physiological functions, for example by suppressing blood pressure and breathing [1]. However, anesthesia is commonly used in preclinical animal experiments because it offers several benefits, such as relief of pain or discomfort in the animals, ease of animal handling, and acquisition of stable signals from the animals. To minimize the unwanted effects of anesthetics on preclinical experiments, a great deal of consideration is required when it comes to choosing anesthetic agents and designing anesthetic protocols, since they may alter the outcome of experiments.
There has been a number of studies that aimed to monitor the altered responses caused by different anesthetic agents [2, 3]. One report showed that ketamine/xylazine/acepromazine cocktail and isoflurane resulted in different hearing sensitivity in the animal cochlea, which may be due to alternation of cochlear function [2]. Another study compared the differences in electrophysiological responses of the visual cortex during ketamine and isoflurane anesthesia [3]. The first study concluded that isoflurane suppresses hearing ability, while ketamine-based anesthetic agent did not; the second showed that isoflurane increased electrophysiology in the frequency range of 10~40 Hz, suppressing the response to visual stimulation, while ketamine increased electrophysiology in the frequency range of 2~4 Hz, enhancing the response to visual stimulation. Therefore, it is necessary to select a suitable anesthetic agent so that unwanted effects do not occur in animal experiments. In another aspect, the use of anesthetic agent minimally affecting the normal physiology will increase the translatability of preclinical studies into clinical studies.
While various kinds of anesthetic agent are available for animal studies, ketamine and isoflurane were tested here, since they are the two main, popular anesthetic agents in animal studies. Ketamine is a general, injectable anesthetic agent, while isoflurane is volatile. Both anesthetic agents have been used to assess female sexual response in preclinical animal studies [4, 5]. In spite of the importance of selecting the most suitable anesthetic agent, however, to the best of our knowledge no research has been conducted to compare the effects of different anesthetics in preclinical sexual arousal study.
To monitor sexual arousal in animal models, laser Doppler flowmetry (LDF) has been used to monitor the change in blood flow in the vaginal wall. However, LDF primarily targets arteries and does not reflect hemodynamic changes in microvessels, where supplying oxygen to the tissue and exchanging nutrients take place [6]. Continuous-wave near-infrared spectroscopy (CW-NIRS) is a technique that can monitor the relative changes in oxy- (OHb), deoxy-(RHb), and total hemoglobin (THb) concentration using NIR light in the range of 650~900 nm. Due to the capability of miniaturization, a NIRS probe is suitable for monitoring hemodynamic changes in the vagina [7]. Moreover, NIRS is sensitive to both arteries and microvessels, which makes it a more suitable tool to monitor vaginal hemodynamics in preclinical studies of female sexual arousal. NIRS can also collect hemodynamic information from both the surface and subsurface of the vaginal wall, while LDF collects information primarily from the surface.
In this study we hypothesized that different anesthetic agents will result in different vaginal hemodynamic response and temperature during sexual arousal in an animal model. To prove this hypothesis, vaginal hemodynamics and temperature of the animals were monitored under either ketamine or isoflurane anesthesia during apomorphine (APO) administration, using an optical probe based on NIRS. Furthermore, we discussed which anesthetic agent is more suitable for studies of female sexual arousal using APO as a sexual stimulation, based on our results and the working mechanisms of APO, isoflurane, and ketamine.
Apomorphine (APO, A4393, Sigma-Aldrich, USA) was administered to each animal through the subcutaneous route at a dose of 80 μg/kg, to induce sexual arousal (APO group). For the same animal, the same volume of saline as the one of APO was administered for comparison (control group). Animals could freely access food and water and were kept under a 12/12 h light/dark cycle. Measurements were performed on female Sprague-Dawley rats (180~220 g,
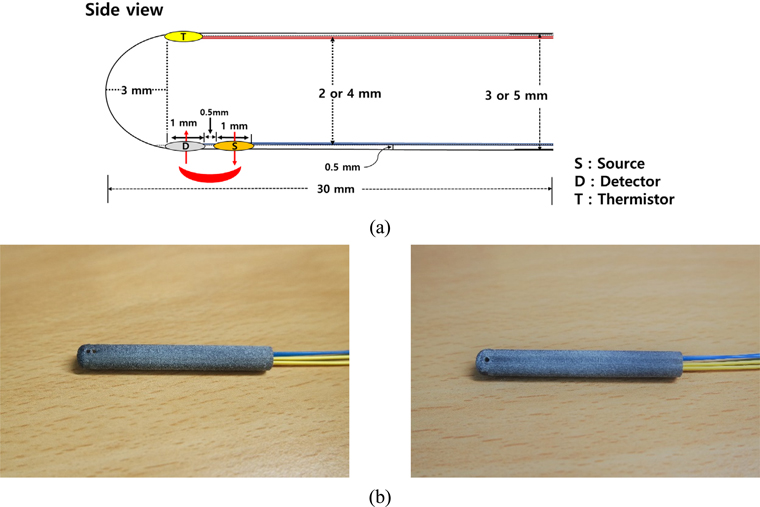
We used a previously developed NIRS based probe to monitor vaginal hemodynamics and temperature changes. The details of the probe can be found in our previous report [7]. Briefly, a cylindrical tube with three holes was utilized as a probe sheath. The probe size was determined considering diameters of 3 mm and 5 mm, depending on the body weight and cylindrical shape of the
The changes in oxyhemoglobin (OHb), deoxyhemoglobin (RHb), and total hemoglobin (THb) concentration induced by APO or saline administration were obtained using the modified Beer-Lambert Law (MBLL). Eq. (1) shows the MBLL for 5 wavelengths (730, 750, 800, 830, and 850 nm), assuming that OHb and RHb are the main chromophores in the wavelength range of 650~900 nm [7]:
where Δ
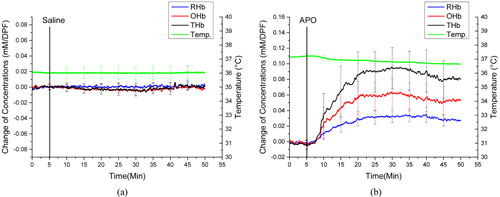
Figures 2(a) and 2(b) show the averaged changes of OHb, RHb, and THb concentrations and temperature from the vaginal wall in the control and APO groups respectively, during ketamine anesthesia. All data from each rat induced by APO were compared to that for the control group. There were no significant changes in OHb, RHb, THb, or temperature in the control group, while all animals in the APO group showed increases in OHb (63 ± 28 μM/DPF), RHb (35 ± 20 μM/DPF), and THb (98 ± 49 μM/DPF). Also, the vaginal temperature dropped gradually from 37.19°C to 36.77°C, post APO administration. These results were presented in our previous report [7].
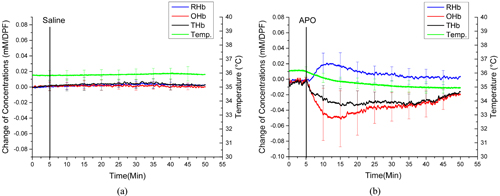
Figures 3(a) and 3(b) show the averaged changes in OHb, RHb, and THb concentrations and temperature from the vaginal wall in the control and APO groups respectively, during isoflurane anesthesia. There were no significant changes in OH b, R H b, T Hb, o r temperature in t he c ontrol group with isoflurane anesthesia, as in the control group with ketamine anesthesia. However, different responses of hemodynamics and vaginal temperature were observed in the isoflurane-anesthetized APO group. Specifically, OHb significantly decreased by 52 ± 76 μM/DPF and gradually returned to the baseline level in the isoflurane-anesthetized APO group, while OHb increased and reached to a plateau in the ketamine-anesthetized APO group. APO administration also caused an increase of RHb by 28 ± 51 μM/DPF and a decrease of THb by 38 ± 30 μM/DPF from the baseline in the isoflurane-anesthetized animals. The vaginal temperature had dropped from 36.14°C to 34.92°C by the end of the experiment in the isoflurane-anesthetized APO group.
IV. DISCUSSIONS AND CONCLUSION
In this study we monitored vaginal hemodynamics and temperature changes caused by APO administration as a means for sexual stimulation in an animal model, under either ketamine or isoflurane anesthesia. To elicit sexual arousal, two main methods are available: nerve stimulation and medication. Nerve stimulation targets the pelvic nerve, sacral nerve, and tibial nerve to induce sexual arousal, but it requires a surgical procedure, which can be a drawback when performed in a rodent model [8]. For the medication, although flibanserin (ADDYI, Sprout Pharmaceuticals) was approved by the FDA for the treatment of pre-menopausal women with hypoactive sexual desire disorder, a great deal of consideration is needed, because it can cause adverse effects such as nausea, dizziness, and drowsiness [9]. Meanwhile, APO, a dopamine agonist that acts in a short time, has shown little adverse effect [10] and has been used in both preclinical and clinical studies to induce sexual arousal [11]. For instance, APO with pelvic nerve stimulation could enhance clitorial and vaginal blood flow in a rabbit model [12], and APO was also used to improve libido in women suffering from sexual desire disorder [13].
It has been reported that vasocongestion (an increase of blood flow and a localized increased blood volume) occurs in the genitalia as a sexual response [14-17]. The results in this study also showed increases of both blood volume (represented by THb increase) and blood flow (OHb increase along with THb increase) during APO administration in the ketamine-anesthetized animals, which agrees with vasocongestion in the genitalia (Fig. 2(b)). However, opposite trends of both OHb and THb were observed in the isoflurane-anesthetized APO group (Fig. 3(b)). This can be explained by interaction between APO-induced vasodilation and blood-pressure changes, depending on anesthetic agent. The side effects of isoflurane include respiratory depression and low blood pressure, which are not found with ketamine [18]. Therefore, when vasodilation occurs by APO administration, a ketamine-anesthetized animal is capable of supplying blood, so that vasocongestion happens in the vaginal wall, shown as increases in OHb and THb. However, APO-induced vasodilation cannot cause vasocongestion in the vaginal wall, due to low blood pressure from the effects of isoflurane anesthesia. As a result, OHb and THb decreased after APO administration in isoflurane-anesthetized animals. These results emphasize the importance of choosing the right anesthetic agent for a given physiological experiment, including this study. Compared to APO-administered groups, saline administration in both isoflurane- and ketamine-anesthetized animals did not show any significant changes in either vaginal hemodynamics or temperature (Figs. 2(a) and 3(a)), which proves that the anesthesia level was well controlled; also, the animals were kept warm using a warm-water pad.
Other than blood volume and flow, the ketamine-anesthetized and isoflurane-anesthetized APO groups also showed a large difference in the change of vaginal temperature from the baseline: a 0.42°C drop in the ketamine group versus a 1.22°C drop in the isoflurane group. It has been shown that sexual arousal causes an increase of genital temperature [19], but the results in this study showed a
One limitation of this study is the lack of an experiment with unanesthetized animals. As the results of this study show, it is very important to minimize the number of factors that can affect the hemodynamics of body. In this case, this includes the anesthesia, since the anesthesia itself causes a change in body hemodynamics. Therefore, employment of an awake-animal model would be best for monitoring vaginal hemodynamics during APO administration; it may be possible, using a rodent restrainer with habituation. However, the adaptation of a restrainer can hardly satisfy the purpose of our study, since the measurement has to be from the vaginal wall, which will be very susceptible to any motion during the measurement. There are many reports using an awake-animal model for brain study, because the head can be held well by using a fixture on it [25-27]. However, it seems infeasible to hold animals for the measurement of vaginal hemodynamics using the probe developed in our previous study.
In summary, we compared vaginal hemodynamics and temperature changes in an APO-administered animal model under either isoflurane or ketamine anesthesia, and found that the vaginal hemodynamics showed a completely different response to APO administration depending on which anesthetic agent was used. These results suggest that the design of a preclinical study to monitor hemodynamic responses under anesthesia should be careful to choose an appropriate anesthetic agent.