All species of sturgeons have complex life histories (Kynard, 1997), requiring tolerance to a large range of environmental conditions at different times of the life cycle, which may be facilitated by physiological mechanisms (Burggren and Randall, 1978). Many species of sturgeons are anadromous, as they spawn in rivers and after a variable length of time, migrate downstream to brackish areas or even the open sea, where they mature until they return to spawn upstream (Martinez-Alvarez, 2002). Ship sturgeon,
Global studies have shown that hatchery reared fish have lower survival rates and provide lower returns to anglers than the wild fish (Heggberget et al., 1992). One of the major problems associated with the viability of restocking is the dramatic level of mortality of newly released individuals (Olla et al., 1998). A study by Berejikian et al. (2000) assumed that perhaps one of the problems of previous attempts to assess the effects of training fingerlings before release is that both trained and untrained fish have been released together. Controlling the induced acclimation to the seawater is a first step to solve a problem. Therefore, study on the physiological condition of juveniles during early stages of life is necessary to understand restocking management (Farabi et al., 2009).
Several studies demonstrated that age and body size have been postulated as determining factors of the salinity tolerance of fish (McEnroe and Cech, 1985; García-Gallego et al., 1998). For example, Mojazi Amiri et al. (2009) reported that early salinity tolerance of two sizes (10 and 30 g) of one-year-old juvenile white sturgeons,
>
Source of Juvenile Acipenser nudiventris and Preliminary Acclimation
Three months old juveniles of
At the beginning of the experiment, a total of 240 juvenile ship sturgeon (average initial weight 6.2 ± 0.13 g) was carefully selected from the stock tanks and directly distributed into 12 fiberglass tanks filled with 100 L of water (20 fish/tank). Each tank was then randomly assigned to one of the three replicates of the four different experimental salinities such as; 0 (control), 4, 8 and 12‰. Fish were not fed throughout the experimental period (10 days). Different salinities were provided by dilution of coastal Caspian brackish water (approximately 12.5‰) with dechlorinated municipal freshwater. Water salinity was determined with a digital salinometer (Cond 330i ⁄ set WTW, Germany) and adjusted daily. Supplemental aeration was provided to maintain the dissolved oxygen at 6.2 ± 0.06 mg L-1, and also water temperature and pH during the experiment were maintained at 23.5 ± 0.08ºC and 7.8 ± 0.02, respectively. Water was replaced in order to prevent accumulation of ammonia and other toxic metabolites in the tanks every day. In addition, siphoning was done every morning in each rearing tank; also, dead fish were removed and recorded daily.
>
Fish sampling and analytical methods
At the end of the trial, five fish per tank were randomly captured, anesthetized with ethylene glycol phenyl ether (200 mg L−1 for 5–10 min), and blood samples were collected from the caudal vein with heparinized syringes for determination of red blood cells (RBC) count, hematocrit (HCT) and hemoglobin (Hb). After the abovementioned measurements with whole blood, plasma was separated by centrifugation at 5,000 g for 10 min and stored at −70℃ for determination of blood biochemical parameters including plasma cortisol, glucose (GLU), potassium (K+) and sodium (Na+). Heparinized microhaematocrit capillary tubes were centrifuged at 16,329.6 g for 5 min in a clinical centrifuge (Hettich-D7200 Tuttlingen, Germany) for HCT. RBC performed by microscope and hemocytometers over cells suspended in Rees-Escher’s solution. Hb was measured using the cyanmethemoglobin method by spectrometer (Cecilce 1020, Germany) at wavelength of 540 nm. Plasma cortisol (mg dl–1) was determined with Radioimmunoassay method with Gama control automatic machine (L.K.B model, Finland). Plasma glucose (mg dl–1) was determined with Ceceil 3021 machine (Technicon Company, USA). Plasma K+ and Na+ values (mM l–1) were measured using a Flamephotometer machine (Corning 480 Model, Jenway Company, England).
Data were analyzed according to one-way ANOVA (Statistix 3.1; Analytical Software, St. Paul, MN, USA) to test the effects of the different water salinities. When a significant treatment effect was observed, an LSD test for multiple comparisons was performed. Treatment effects were considered at
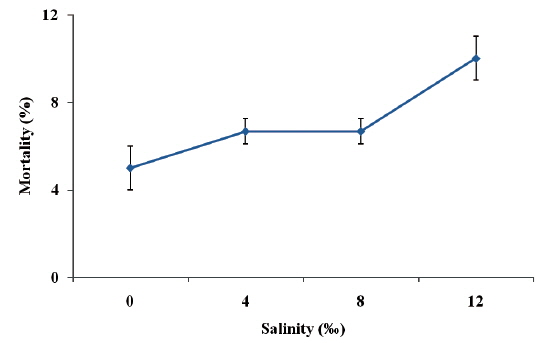
Fish exposure to 0‰ salinity presented normal swimming and did not show any external signs of distress. Under 4‰ and 8‰ salinities, ship sturgeon still did not show any external signs of distress. However, when fish were exposed to 12‰ salinity, there was a marked reduction in swimming speed. The highest percentage mortality (10.0%) was observed in juvenile ship sturgeon exposed to 12‰, while the lowest (5.0%) was recorded in the control (Fig. 1), and there were no significant differences between treatments (
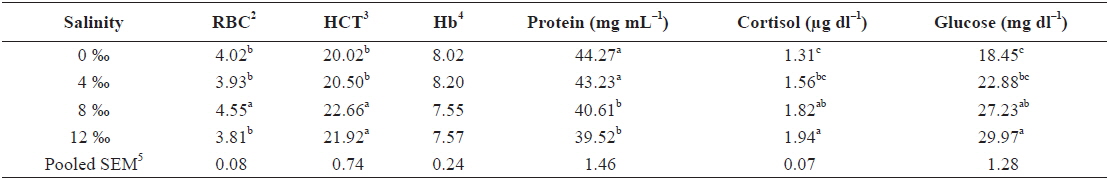
Table 1 shows the hematological parameters of fish under different salinity levels. RBC of fish exposed to 8‰ salinity was significantly higher than those of fish subjected to 0‰, 4‰ and 12‰ salinities (
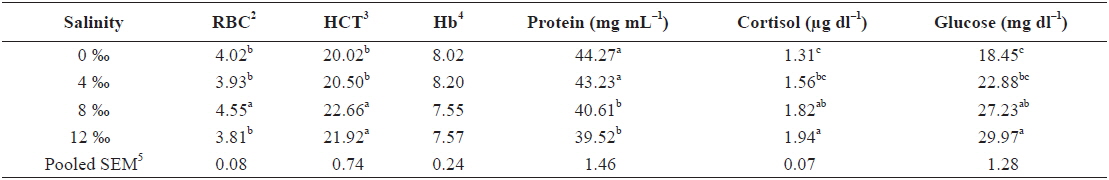
Effects of different salinity levels on hematological parameters of juvenile ship sturgeon Acipenser nudiventris after 10 days1
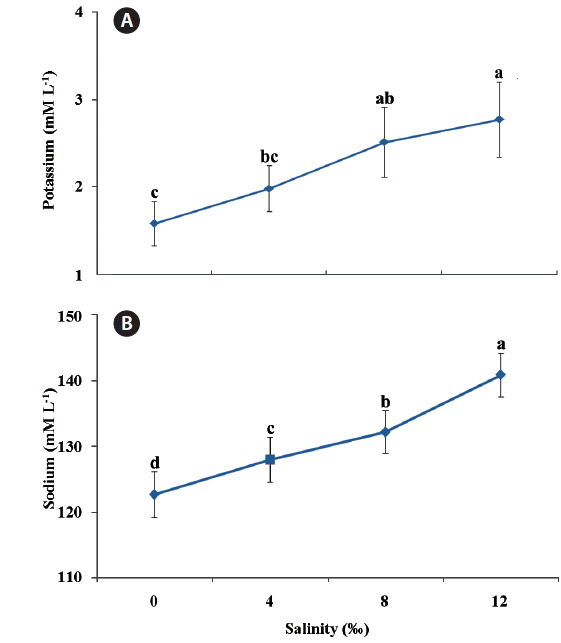
Plasma potassium (Fig. 2a) and sodium (Fig. 2b) values increased significantly by increment of salinity levels (
In the present study, Mortality among fish transferred to brackish water is inversely related to their ability to osmoregulate and depending on fish size, such transfer often results in high mortality rates (Cataldi et al., 1999). Our results showed that 3-month old juvenile ship sturgeons were able to survive direct transfer to brackish water (4, 8 and 12‰) and little mortality was detected among the individuals subjected to these salinity levels.
The effect of cortisol on osmoregulatory parameters has been amply studied in teleosts (Hegab and Henque, 1984). Its primary action seems to be the stimulation of Na+/K+-ATPase activity. In the current study, the trend of increasing levels of cortisol at higher salinity in ship sturgeon indicates that for this chondrostean, the role of cortisol must be similar to that in teleosts. Besides, the increase of plasma cortisol value is considered to be a primary indicator of stress response (Cataldi et al., 1998). Cleary et al. (2002) showed that plasma cortisol levels of stressed snapper is possible that following the initial osmotic challenge and associated immediate serum chemistry perturbations, and subsequent restoration of homeostasis, that stress hormones influenced plasma electrolyte flux. It is known that stress induced hormonal responses, such as elevated levels of plasma cortisol and catecholamines, lead to osmotic imbalances in fish subjected to hypertonic and hypotonic environments (Pickering and Pottinger, 1995). The trend for the cortisol level to rise in response to growing environmental salinity should, like a hyperglycemia-causing hormone, raise the plasma glucose value. Similarly, we found such rise in our results. Previous studies of this issue are contradictory, showing both a rise (Assem and Hanke, 1979; Bashamohideen and Parvatheswararao, 1972) and a fall (Krumschnabel and Lackner, 1993; Soengas et al., 1991) in glucose value during seawater adaptation. There appears to be a high glucose demand in order to supply the energy by osmoregulatory mechanisms (Krumschnabel and Lackner, 1993; Plaut, 1998), whereupon glyconeogenesis even increases (Jürss and Bittorf, 1990). The greater use of glucose could mask the plasma glucose increase prompted by the cortisol.
The decline found in plasma proteins during increase of salinity could also be accounted for by the high osmoregulatory energy demand. Huang et al. (2006) noted that as environmental salinity increased, fish consumed more energy, while glucose and lipids provided the energy required for metabolism. Therefore, when the available food source lacks sufficient energy, protein in the feed would be utilized as energy source (Lin, 1999).
The HCT and RBC increased with increasing salinity levels up to 8‰ salinity; afterwards, they subsequently decreased as the salinity increased further. The change in environmental salinity can be attributed to changes in the water content in the blood (Plaut, 1998). Thus, at the beginning of exposure to a hyperosmotic environment, the fish would lose water passively, and thereby undergo increases in the concentrations of blood-cell elements. Afterwards, the compensatory increase in water ingestion would provide a transitory dilution of the blood parameters. Finally, these would return to initial values as a result of the rest of the osmoregulatory mechanisms, which act to re-establish the extracellular volume (Martinez-Alvarez et al., 2002).
If the internal perturbation of the fish, either directly or as a result of alterations of the environment, overwhelms the physiological mechanisms of the animal for response and adaptation to new conditions can be threatened and death is resulted (Martinez Alvarezi et al., 2002). Therefore, anadromous fish must develop complex osmoregulatory mechanisms to survive successfully both in hypoosmotic environments (e.g. rivers) and hyperosmotic environments (e.g. estuaries and open sea). In previous investigations, it was noted that broodstock and juvenile acipenserids of euryhaline species stabilize the plasma osmolarity and ionic concentration after transferred from freshwater more slowly, for the duration of 7-10 days (Krayushkina, 1983a) than teleost fish in particular salmonids, for duration 3-5 days to seawater (Krayushkina, 1983b).
In the present study plasma potassium (K+) and sodium (Na+) values in ship sturgeon significantly increased by increment of salinity levels. In agreement with our results, Farabi et al. (2009) noted an increase in plasma potassium (K+) and sodium (Na+) values in ship sturgeon with increasing water salinity. Likewise, Lebreton and Beamish (1998) found that the plasma concentrations of (K+) and (Na+) ions in
This observation indicates that a difference exists in the ion levels between fish acclimated to brackish water and freshwater. These differences might be due to species-specific morphophysiological mechanisms for salinity adaptation and tolerance, which would be directly related to the natural history of this species. In conclusion, these results indicated that in natural environments, juvenile ship sturgeon,